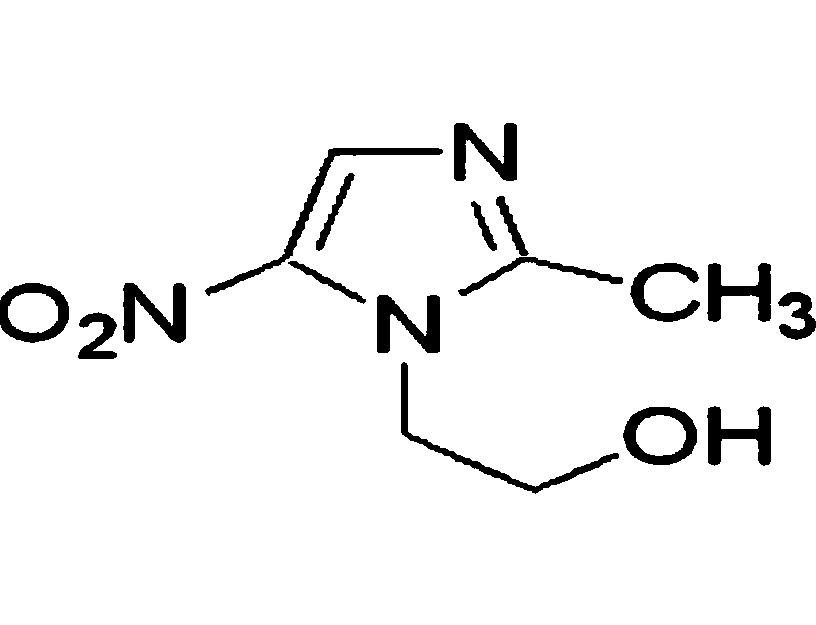
Metronidazole composition freeze-dried disintegrating tablets for vaginas and preparation method thereof
A technology of metronidazole and composition, applied in the field of metronidazole composition vaginal freeze-dried disintegrating tablet and its preparation field, can solve the problems of inconvenient transportation and storage, irritation of inflammatory mucosa, influence on clinical application, etc. Effectiveness of availability, less vaginal irritation, and shorter dissolution time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Prescription: 1000 tablets
[0043]
[0044] Dissolve the prescription amount of medium substituted hydroxypropyl-β-cyclodextrin in purified water of 80% of the total amount of prescription water, heat to 55°C while stirring, slowly add the prescription amount of metronidazole, and continue stirring for 10 Hours, after the medium-substituted hydroxypropyl-β-cyclodextrin clathrates the metronidazole, add the prescribed amount of mannitol and sucralose, and dissolve the prescribed amount of gelatin in purified water with 20% of the prescribed water amount , heated until completely dissolved, combined the above two solutions and stirred evenly, adjusted the pH value to 5.5-6.5 with sodium bicarbonate, according to the specifications of metronidazole composition vaginal freeze-dried disintegrating tablets, after determining the loading amount, the combined The medicinal liquid is divided into medicinal dishes according to the amount of loading, and put into a freeze-dryi...
Embodiment 2
[0046] Prescription: 1000 tablets
[0047]
[0048] Dissolve the prescription amount of medium substituted hydroxypropyl-β-cyclodextrin in purified water of 80% of the total amount of prescription water, heat to 55°C while stirring, slowly add the prescription amount of metronidazole, and continue stirring for 10 Hours, after the medium-substituted hydroxypropyl-β-cyclodextrin clathrates metronidazole, add the prescribed amount of mannitol and sucralose, and dissolve the prescribed amount of gelatin in purified water with 20% of the prescribed water consumption. Heat until completely dissolved, combine the above two solutions and stir evenly, adjust the pH value to 5.5-6.5 with sodium bicarbonate, according to the specifications of metronidazole composition vaginal freeze-dried disintegrating tablets, after determining the filling amount, the combined medicine The solution is divided into medicine-containing dishes according to the content, and put into a freeze-drying box ...
Embodiment 3
[0050] Prescription: 1000 tablets
[0051]
[0052] Dissolve the prescription amount of medium substituted hydroxypropyl-β-cyclodextrin in purified water of 80% of the total amount of prescription water, heat to 55°C while stirring, slowly add the prescription amount of metronidazole, and continue stirring for 10 Hours, after the medium-substituted hydroxypropyl-β-cyclodextrin clathrates metronidazole, add the prescribed amount of mannitol and sucralose, and dissolve the prescribed amount of gelatin in purified water with 20% of the prescribed water consumption. Heat until completely dissolved, combine the above two solutions and stir evenly, adjust the pH value to 5.5-6.5 with sodium bicarbonate, according to the specifications of metronidazole composition vaginal freeze-dried disintegrating tablets, after determining the filling amount, the combined medicine The solution is divided into medicine-containing dishes according to the content, and put into a freeze-drying box to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com