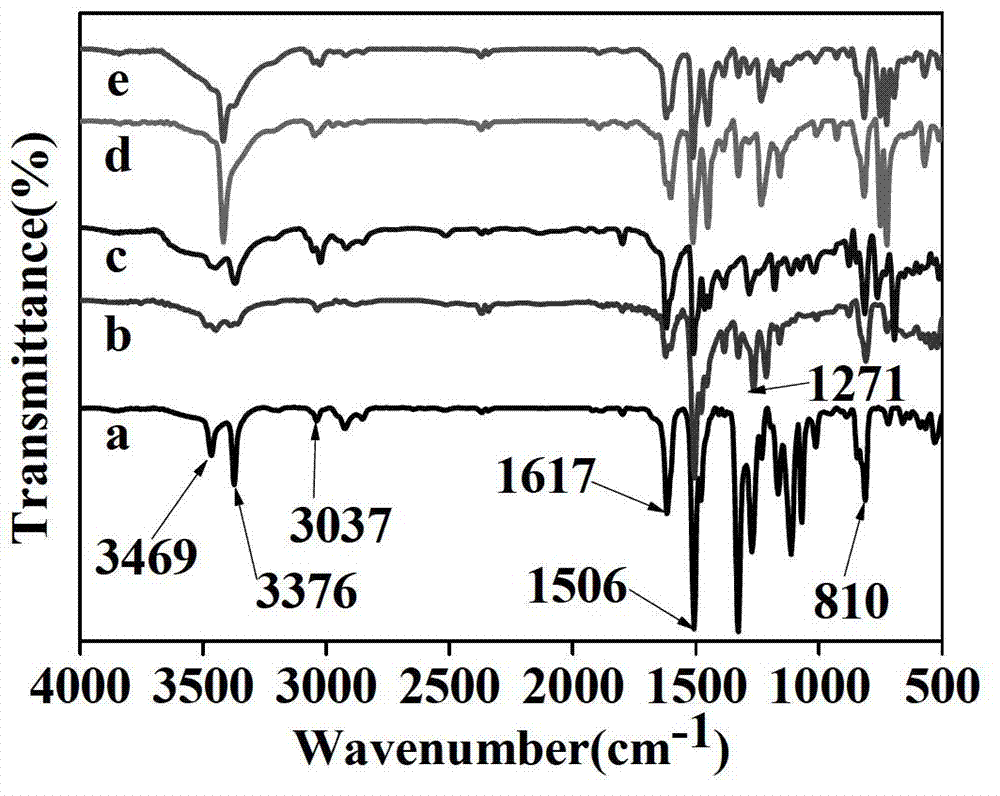
Novel function diamine monomer containing carbazole and large conjugated structure and preparation method and application thereof
A conjugated structure, diamine monomer technology, applied in the field of material science, can solve problems such as performance needs to be further improved, single variety, etc., and achieve the effects of high yield, easy purification, and simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] Wherein, the preparation method of the novel functional diamine monomer containing carbazole and large conjugated structure as shown in general structural formula I or II comprises the following steps:
[0029] (1) Use the active hydrogen in the dihalogenated carbazole monomer carbazole to graft a large conjugated structure to obtain a large conjugated system monomer containing two halogen atoms. The large conjugated structure containing two halogen atoms has the following structural features:
[0030]
[0031] Where X can be fluorine, chlorine, bromine or iodine.
[0032] (2) Utilize p-aminophenylboronic acid to react with two halogen atoms of the macroconjugated system monomer containing two halogen atoms described in step (1) by Suzuki reaction to obtain the compound shown in general structural formula I or II A new functional diamine monomer with a carbazole structure and a large conjugated system.
[0033] The preparation method of the novel functional diamine...
Embodiment 1
[0046] N 1 -(4-(3,6-bis(4-(trifluoromethyl)phenyl)-9H-carbazol-9-yl)phenyl)-N 1 Synthesis of -(4-aminophenyl)benzene-1,4-diamine:
[0047]
[0048] (1) Synthesis of intermediate 3,6-dibromo-9-(4-nitrophenyl)-9H-carbazole:
[0049] 32.5g (0.1mol) of 3,6-dibromocarbazole (3,6-dibromo-9H-carbazole), 11.2g (0.3mol) of potassium tert-butoxide (t-BuOK) and 21.165g (0.10mol) of Add fluoronitrobenzene (1-fluoro-4-nitrobenzene) into a 500ml three-necked flask, use N,N-dimethylformamide (DMF) as a solvent, stir magnetically and pass argon gas, heat and reflux in an oil bath at 100°C for 64h , the reaction solution was poured into ice water, suction filtered to obtain a khaki solid, and the yellow solid was recrystallized to obtain a light yellow crystal, which was dried in vacuo to obtain a khaki intermediate product 3,6-dibromo-9-(4-nitrophenyl )-9H-carbazole 41.5g.
[0050] (2) Synthesis of 9-(4-nitrophenyl)-3,6-bis(4-(trifluoromethyl)phenyl)-9H-carbazole:
[0051] 8.9218g (0....
Embodiment 2
[0059] N 1 -(4-(3,6-bis(4-fluorophenyl)-9H-carbazol-9-yl)phenyl)-N 1 Synthesis of -(4-aminophenyl)benzene-1,4-diamine:
[0060]
[0061] (1) Synthesize the intermediate 3,6-dibromo-9-(4-nitrophenyl)-9H-carbazole according to Example 1;
[0062] (2) Synthesis of 4-(3,6-dibromo-9H-carbazol-9-yl)benzolamine:
[0063] Add 32.5g (0.1mol) of 3,6-dibromo-9-(4-nitrophenyl)-9H-carbazole into a 250ml three-necked flask, add 150ml of absolute ethanol, magnetically stir and argon, and heat the oil bath to 70°C Finally, add 10%wt palladium carbon 0.2g, and gradually drop 50ml hydrazine hydrate, after reflux reaction 10h, suction filtration after cooling, collect beige solid, obtain beige product 4-(3 ,6-dibromo-9H-carbazol-9-yl)benzonamine 39.9g.
[0064] (3) Synthesis of intermediate N-(4-(3,6-dibromo-9H-carbazol-9-yl)phenyl)-4-nitro-N-(4-nitrophenyl)benzonamine:
[0065] 41.611g (0.1mol) 4-(3,6-dibromo-9H-carbazol-9-yl) benzonamine, 45.574g (0.03mol) cesium fluoride (CsF) and 42....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com