Device and method for performing olefin gaseous phase epoxidation by using industrial hydrogen peroxide
A technology of hydrogen peroxide and epoxidation, applied in chemical recovery, organic chemistry, etc., can solve the problems of low economic benefit, low raw material utilization rate, reduced reaction efficiency, etc., to overcome the rapid deactivation of catalysts and the adaptability of raw materials Strong, overcoming the effect of low efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1 (comparative example):
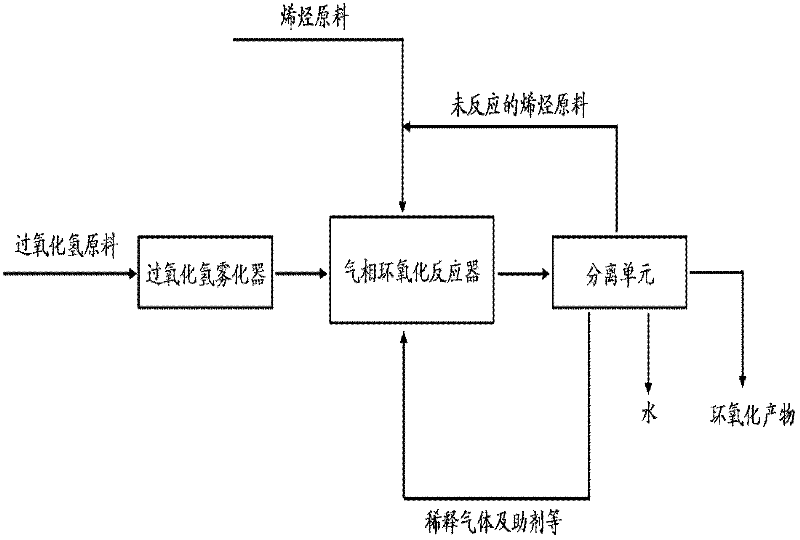
[0052]Referring to the public document Ind. Eng. Chem. Res. 47 (2008) 2086-2090, the method of vaporizing hydrogen peroxide with a falling film evaporator was used to design a reaction process for gas-phase epoxidation of propylene. The process includes: hydrogen peroxide falling film evaporator, small fixed-bed gas-phase epoxidation reactor and gas-liquid separation tank. The reactor shell is made of metal material, and the inner wall contacting hydrogen peroxide is passivated with nitric acid. The constant temperature zone of the reactor is filled with 5g of catalyst. The catalyst is TS-1 zeolite extruded from silica sol. The grain size of TS-1 zeolite is 1×2×6 μm, and the Si / Ti molar ratio is about 33. 50% by weight. Above and below the catalyst bed are packing layers, and the packing layers are inert ceramic balls. The main process is: after the raw material hydrogen peroxide is vaporized by the falling film evaporator, it en...
Embodiment 2
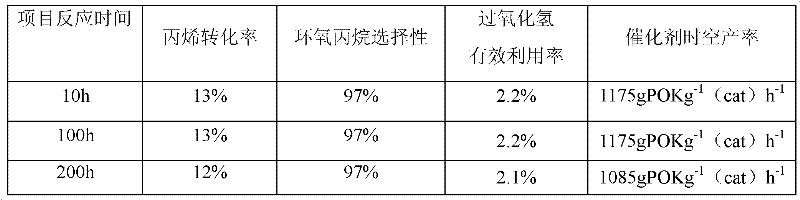
[0058] Example 1 was repeated, but the falling film evaporator used for hydrogen peroxide vaporization was changed to an ultrasonic nebulizer. Adjust the power of the ultrasonic atomization device to maintain the hydrogen peroxide atomization rate at 300ml / min. After measurement, the particle size of hydrogen peroxide droplets after atomization is 1-3 μm, and the concentration is about 50wt% (15mol / L), which is the same as the concentration of the raw material solution, so the propylene / molar ratio is 1 / 5, and the epoxidation reaction results for:
[0059]
[0060] This example verifies that the ultrasonic atomization method does not decompose hydrogen peroxide and can avoid the problem of selective vaporization.
Embodiment 3
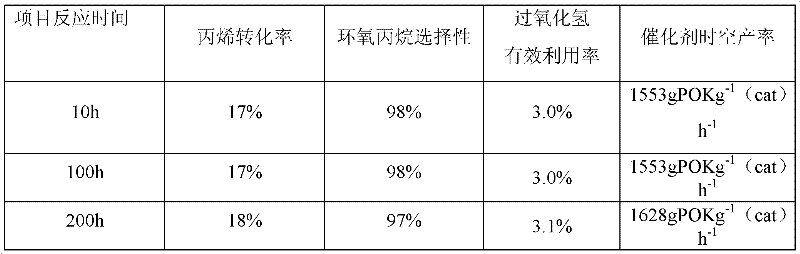
[0062] Example 1 was repeated, but the falling film evaporator used for hydrogen peroxide vaporization was changed to a plasma electrostatic atomizer. Said plasma electrostatic atomizer mainly consists of several capillary columns and electrostatic generators. The capillary column is made of nitric acid passivated stainless steel, with an inner diameter of 1mm and a length of 0.5m. Adjust the feed pump flow rate to maintain the hydrogen peroxide atomization rate at 300ml / min. After measurement, the concentration of hydrogen peroxide solution after atomization is about 50wt% (15mol / L), identical with the concentration of raw material liquid, then propylene / hydrogen peroxide mol ratio is 1 / 5, and epoxidation reaction result is:
[0063]
[0064] This example verifies that the plasma electrostatic atomizer does not decompose hydrogen peroxide and can avoid the problem of selective vaporization.
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| crystal size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com