Nifedipine framework sustained-release pellets and preparation method and application thereof
A technology for nifedipine and sustained-release pellets, applied in the field of medicine, can solve the problems of poor reproducibility between batches, low yield of pellets, and poor hardness, and achieves improved bioavailability, improved bioavailability, and improved release. effect of behavior
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
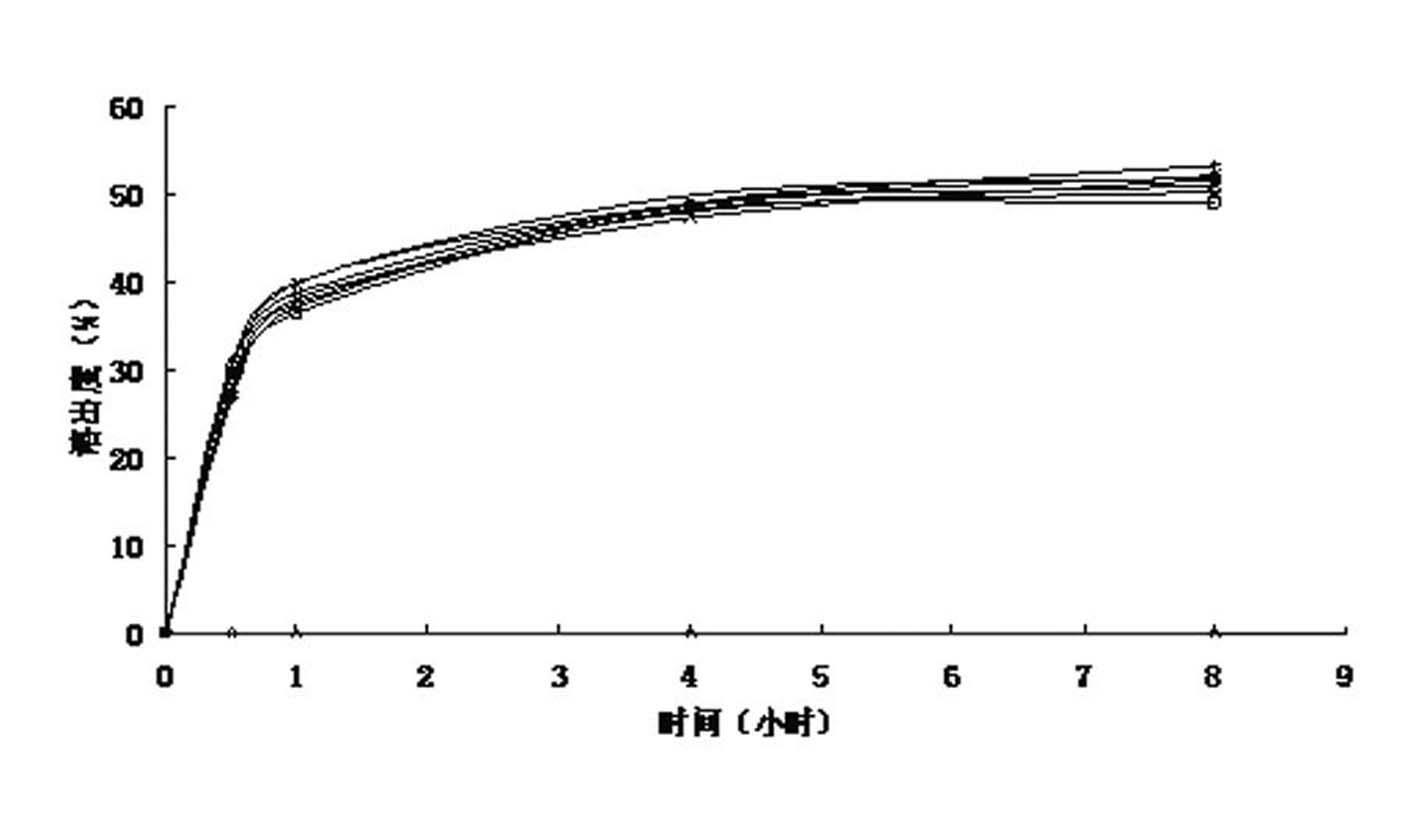
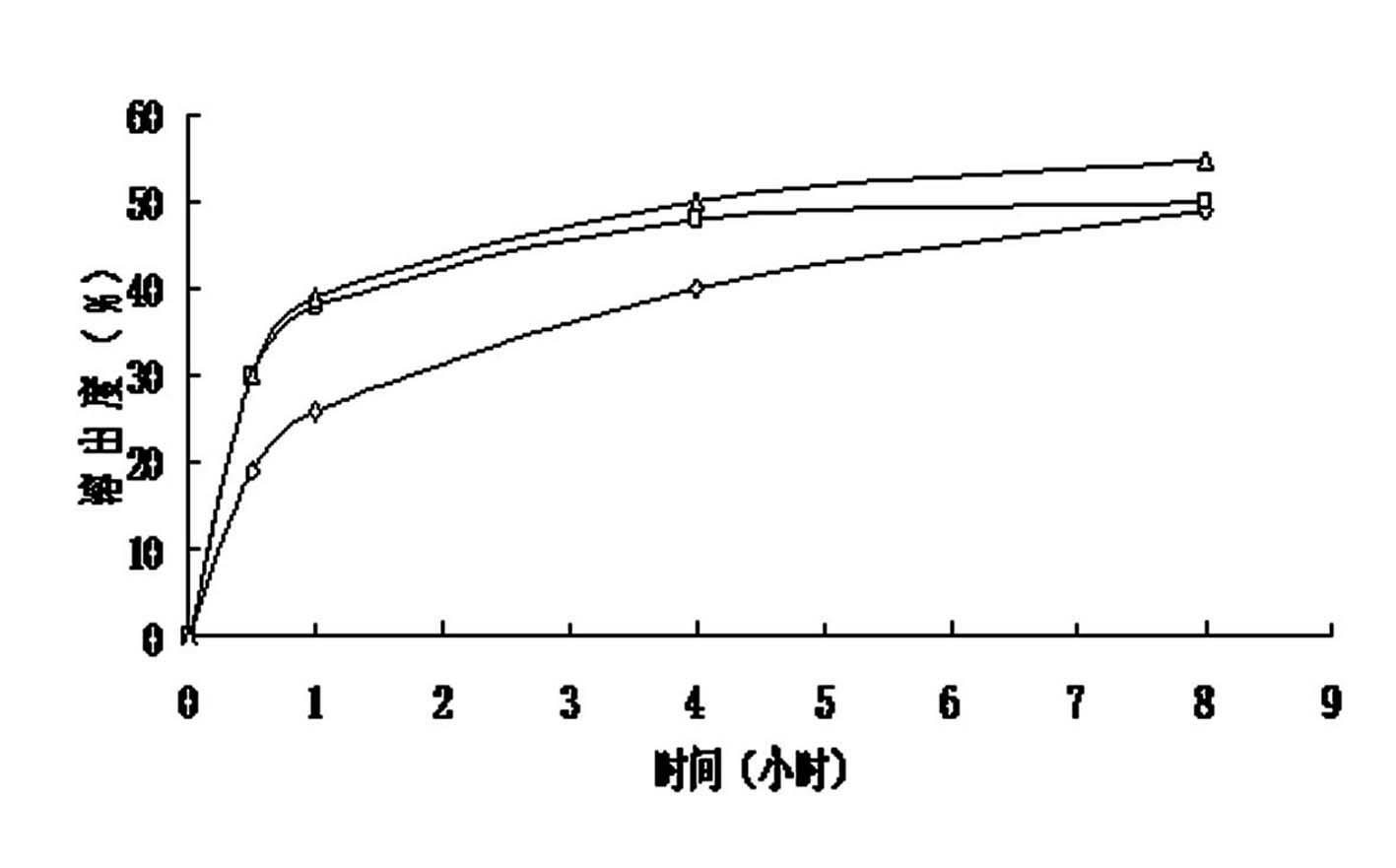
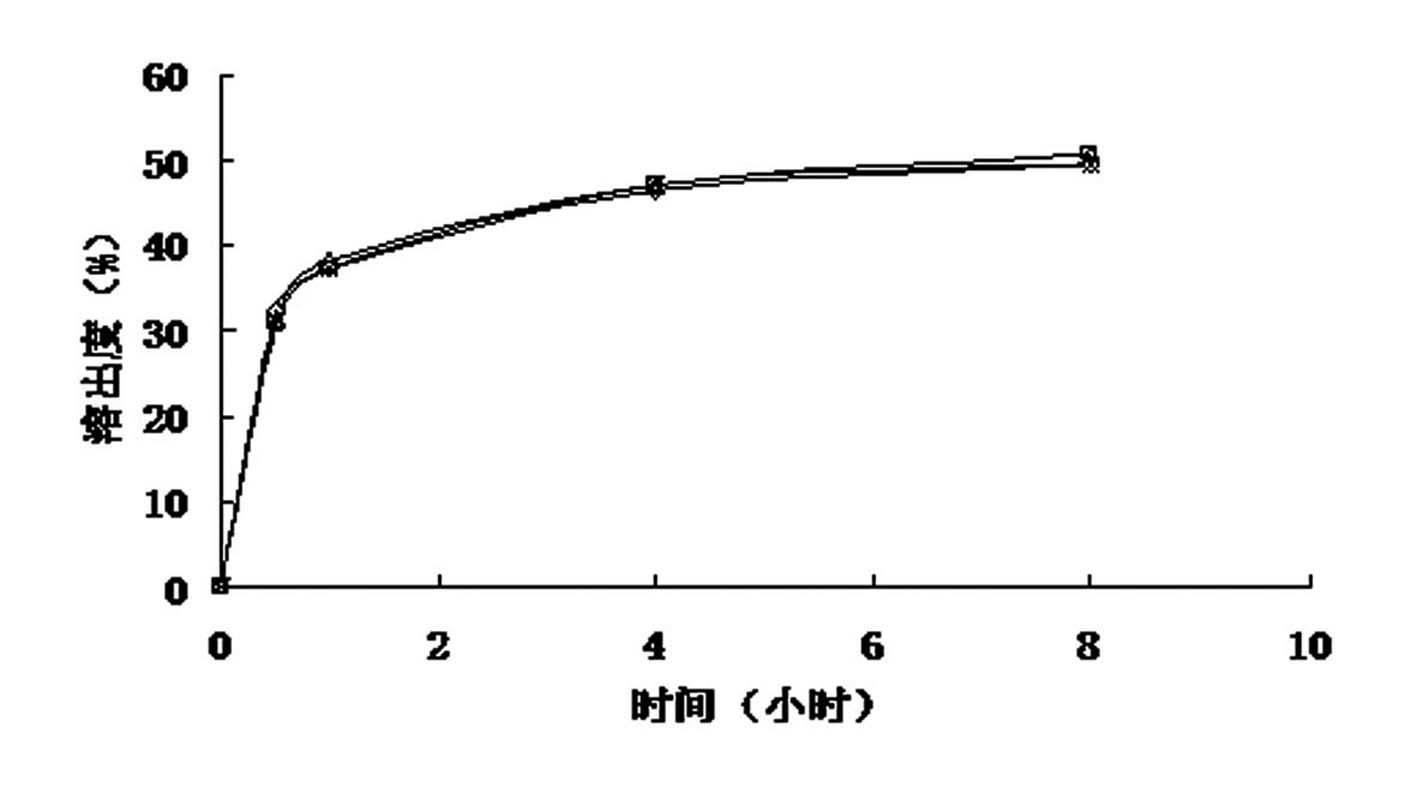
Image
Examples
Embodiment 1
[0041] The raw materials of the liquid co-grind are as follows: 500g of nifedipine, 40g of hypromellose E5, 10g of PEG6000, and 500ml of water;
[0042] The raw materials of the pellets are as follows: Nifedipine liquid co-grind 26g, microcrystalline cellulose 15g, mannitol 57g, sodium lauryl sulfate 12g, sodium carboxymethyl starch 2g;
[0043] The raw materials for coating are as follows: 500g of nifedipine matrix pellets, 15g of brown Opadry, 100ml of water;
[0044] The specification is 20mg / capsule.
[0045] Its preparation method is as follows:
[0046] (1) Preparation of liquid co-grind of nifedipine: 500g of nifedipine was suspended in an aqueous solution of a hydrophilic carrier, which was prepared by mixing 40 g of hypromellose E5, 10 g of PEG6000 and 500 ml of water. into, the concentration of the aqueous solution of the hydrophilic carrier is 10% (weight / volume). Put the suspension in a basket mill, and grind it for 4 hours under the condition of a grinding ...
Embodiment 2
[0051] The raw materials of the liquid co-grind are as follows: 400g of nifedipine, 30g of hypromellose E5, 600ml of water;
[0052] The raw materials of the pellets are as follows: 35g of nifedipine liquid co-grind, 18g of microcrystalline cellulose, 54g of lactose, 12g of sodium lauryl sulfate, 3g of croscarmellose sodium;
[0053] The raw materials for coating are as follows: 500g of nifedipine matrix pellets, 20g of brown Opadry, 133.33ml of water;
[0054] The specification is 20mg / capsule.
[0055] Its preparation method is as follows:
[0056] (1) Preparation of nifedipine liquid co-grind: 400g of nifedipine was suspended in an aqueous solution of a hydrophilic carrier, which was prepared by mixing 30 g of hypromellose E5 and 600 ml of water. The concentration of propylmethylcellulose E5 aqueous solution is 5 (w / v)%. The suspension is placed in a basket mill and ground for 4 hours at a grinding speed of 1500 rpm to obtain the product.
[0057] (2) Preparation of n...
Embodiment 3
[0061] The raw materials of the liquid co-grind are as follows: 200g of nifedipine, 30g of hypromellose E5, 30g of compressible starch, 120ml of ethanol, and 480ml of water;
[0062] The raw materials of the pellets are as follows: Nifedipine liquid co-grind 40g, microcrystalline cellulose 20g, mannitol 54g, sodium lauryl sulfate 15g, sodium carboxymethyl starch 2g, sodium bicarbonate 2g;
[0063] The raw materials for coating are as follows: 500g of nifedipine matrix pellets, 25g of titanium dioxide, and 166.67ml of water;
[0064] The specification is 20mg / capsule.
[0065] Its preparation method is as follows:
[0066] (1) Preparation of nifedipine liquid co-grind: 200g of nifedipine was suspended in ethanol aqueous solution of hydrophilic carrier, which was composed of 30g hypromellose E5, 30g compressible starch, It is prepared by mixing 480ml of water and 120ml of ethanol. The concentration of the aqueous solution of the hydrophilic carrier is 10% (weight / volume). The ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com