Ammonia circulative preparation method for cobaltous oxalate
A cobalt oxalate and ammonia recycling technology, applied in the preparation of carboxylate, organic chemistry, etc., can solve the problems of difficult equipment maintenance, poor uniformity, long process, etc., to achieve a circular economy, avoid emission problems, and reduce production costs. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
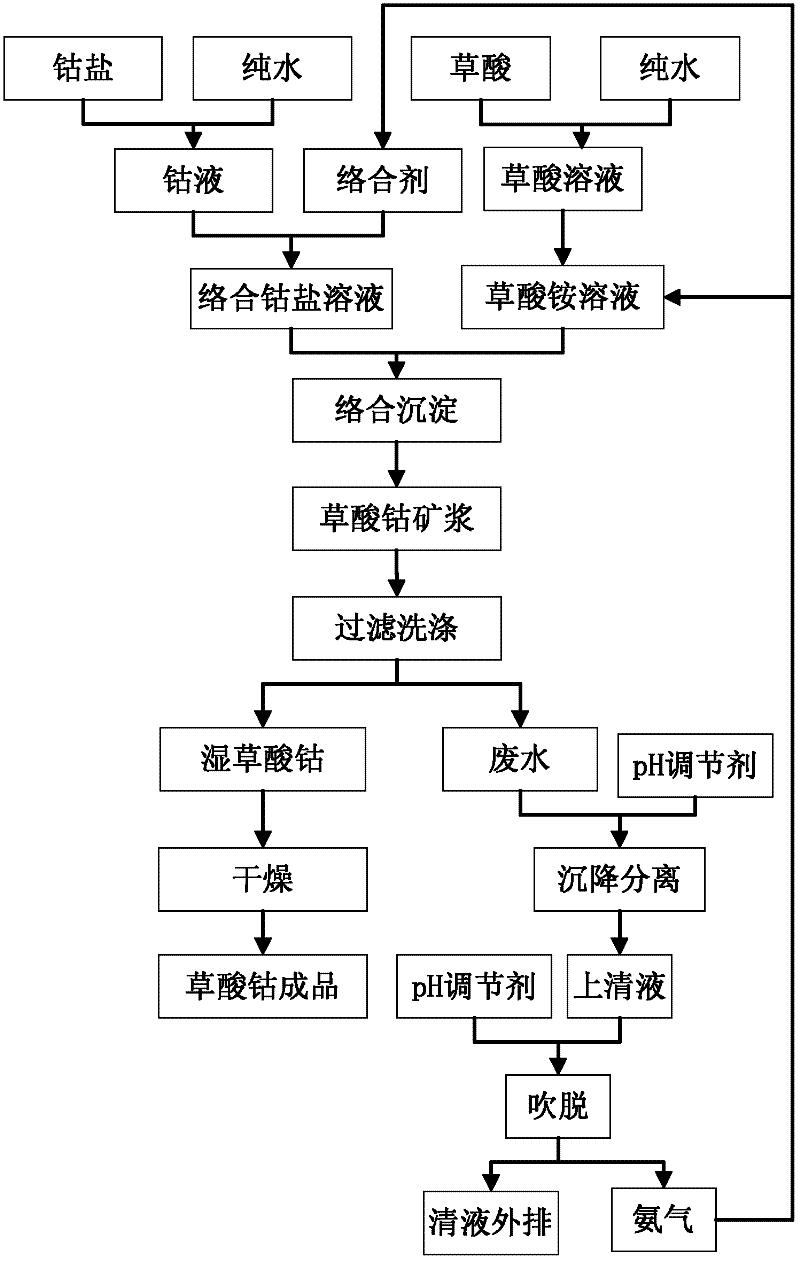
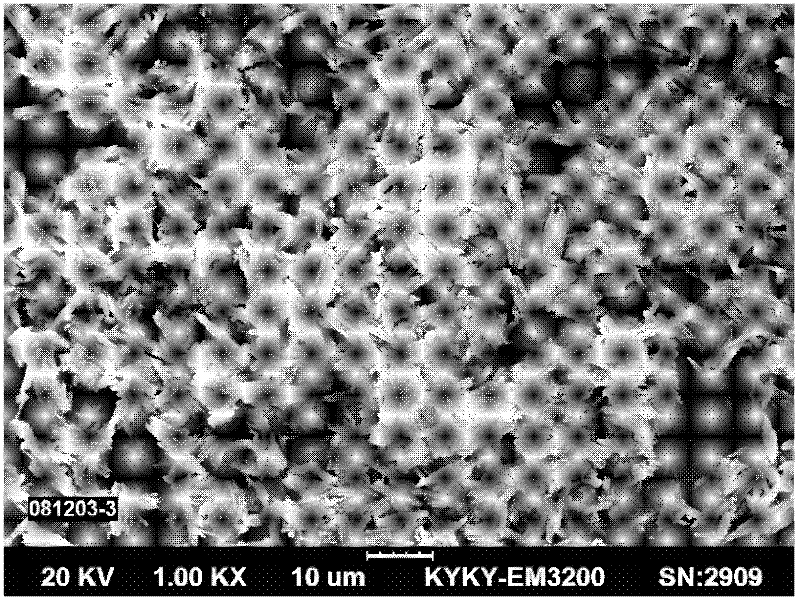
[0024] Such as figure 1 As shown, cobalt chloride salt is used to dissolve to obtain 30-90g / L cobalt liquid, and one or more of ammonia water, EDTA, and ammonium citrate are added to the above cobalt liquid according to 5%-30% of the cobalt mass, as Complex cobalt salt solution. Dissolving oxalic acid to obtain 50-100g / L oxalic acid solution, absorbing ammonia gas to obtain ammonium oxalate solution. Add excess 5%-25% ammonium oxalate solution and complex cobalt salt solution to the reactor by co-current or forward-flow feeding method, control the synthesis reaction temperature to be 30°C-70°C, and the reaction pH to be 2.0-3.0, and stir The speed is 400 rpm -1000rpm, and the cobalt oxalate slurry is continuously produced by the complexation-homogeneous precipitation method; the cobalt oxalate slurry is filtered and washed by a belt filter, and the wet cobalt oxalate is dried to obtain the finished cobalt oxalate product with a loose ratio of 0.23 g / cm 3 , the Fisherman's p...
Embodiment 2
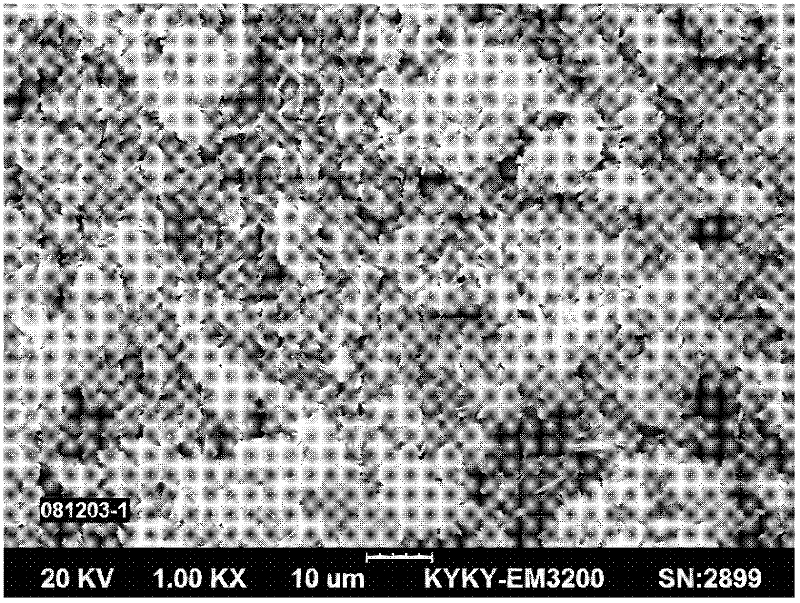
[0026] Such as figure 2 As shown, use cobalt sulfate salt to dissolve to obtain 30-90g / L cobalt liquid, and add one or more of ammonia water, EDTA, and ammonium citrate to the above cobalt liquid according to 10%-30% of the cobalt mass, as a complex cobalt salt solution. Dissolving oxalic acid to obtain 50-100g / L oxalic acid solution, absorbing ammonia gas to obtain ammonium oxalate solution. Add excess 5%-25% ammonium oxalate solution and complex cobalt salt solution to the reactor by co-current or forward-flow feeding method, control the synthesis reaction temperature to be 30°C-70°C, and the reaction pH to be 2.0-3.0, and stir The speed is 400 rpm -1000rpm, and the cobalt oxalate slurry is continuously produced by the complexation-homogeneous precipitation method; the cobalt oxalate slurry is filtered and washed by a belt filter, and the wet cobalt oxalate is dried to obtain the finished product of cobalt oxalate with a loose ratio of 0.18 g / cm 3 , the Fisherman's parti...
Embodiment 3
[0028] Such as figure 1 As shown, cobalt nitrate salt is used to dissolve to obtain 30-90g / L cobalt liquid, and one or more of ammonia water, EDTA, and ammonium citrate are added to the above-mentioned cobalt liquid according to 10%-25% of the cobalt mass, as a complex cobalt salt solution. Dissolving oxalic acid to obtain 60-100g / L oxalic acid solution, absorbing ammonia gas to obtain ammonium oxalate solution. Add excess 10%-20% ammonium oxalate solution and complex cobalt salt solution to the reaction kettle by co-current or forward-flow feeding method, control the synthesis reaction temperature to be 30°C-70°C, and the reaction pH value to be 2.0-3.0, stir The speed is 500 rpm -1000rpm, and the cobalt oxalate slurry is continuously produced by the complexation-homogeneous precipitation method; the cobalt oxalate slurry is filtered and washed by a belt filter, and the wet cobalt oxalate is dried to obtain the finished cobalt oxalate product with a loose ratio of 0.3 g / cm ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com