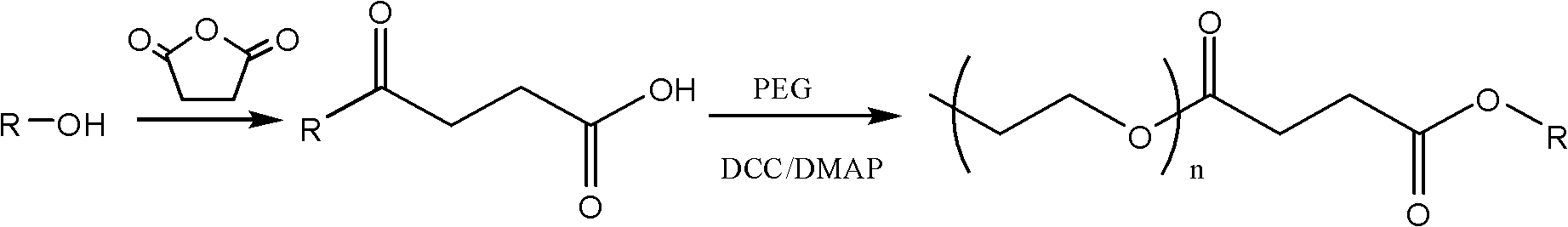
Docetaxel nano preparation and preparation method thereof
A technology of docetaxel and nano-formulations, which is applied in the directions of pill delivery, pharmaceutical formulations, and inactive medical preparations, can solve the problems of low bioavailability, unsatisfactory stability and storage stability, etc. Good stability, long-term release, good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] This example provides a nano freeze-dried powder injection of docetaxel, which is prepared by the following steps:
[0032] Weigh 15 mg of docetaxel and 100 mg of polybenzyl glutamate with a weight average molecular weight of 64,000, add them to 10 ml of organic solvent ethyl acetate, stir and dissolve, and take 3 mg of vitamin E polyethylene glycol amber Ester (self-made, using PEG1000), dissolved in 150ml ultra-pure water, and prepared as an aqueous solution; the organic phase was added to the aqueous solution, and the O / W The colostrum is then subjected to high-speed shear (shear rate range 10000-12000rpm) for 0.1-5min to prepare a stable emulsion. Then evaporate under reduced pressure at room temperature to remove ethyl acetate to obtain a colloidal solution of nanoparticles. After the colloidal solution is centrifuged at 11500 rpm for 30 minutes at 4°C, the supernatant is discarded, and the precipitate is washed 3 times with ultrapure water to obtain Doxyl Taxel n...
Embodiment 2
[0035] This example provides a nano freeze-dried powder injection of docetaxel, which is prepared by the following steps:
[0036] Weigh 15 mg of docetaxel and 100 mg of polybenzyl glutamate with a weight average molecular weight of 64,000, add it to 10 ml of organic solvent methylene chloride, stir and dissolve, and use it as the organic phase; another 15 mg of cholesterol polyethylene glycol succinic acid Esters (self-made, using PEG 2000), dissolved in 150ml ultrapure water, and prepared as an aqueous solution; the organic phase was added to the aqueous solution, and the O / W The colostrum is then subjected to high-speed shear (shear rate range 10000-12000rpm) for 0.1-5min to prepare a stable emulsion. Then dichloromethane was volatilized under reduced pressure at room temperature to obtain a colloidal solution of nanoparticles. After the colloidal solution was centrifuged at 11,500 rpm for 30 minutes at 4°C, the supernatant was discarded, and the precipitate was washed 3 ti...
Embodiment 3
[0039] This example provides a nano freeze-dried powder injection of docetaxel, which is prepared by the following steps:
[0040] Weigh 25 mg of docetaxel and 375 mg of poly(lactic-co-glycolic acid) copolymer with a weight average molecular weight of 15,000, add it to 25 ml of organic solvent ethyl acetate, stir and dissolve, and take 1.9 mg of vitamin E polyethylene glycol Succinate (self-made, using PEG 1000), dissolved in 375ml ultrapure water, was prepared as an aqueous solution; the organic phase was added to the aqueous solution, and O / W colostrum, and then quickly pour the formed O / W colostrum into the SPG membrane emulsifier, and pass through the membrane 3 to 5 times under the pressure of 0.6-1.8Mpa. Then, the organic solvent was evaporated under reduced pressure at room temperature to obtain a colloidal solution of nanoparticles. After the solution was centrifuged at 11500 rpm for 30 minutes at 4°C, the supernatant was discarded, and the precipitate was washed 3 ti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com