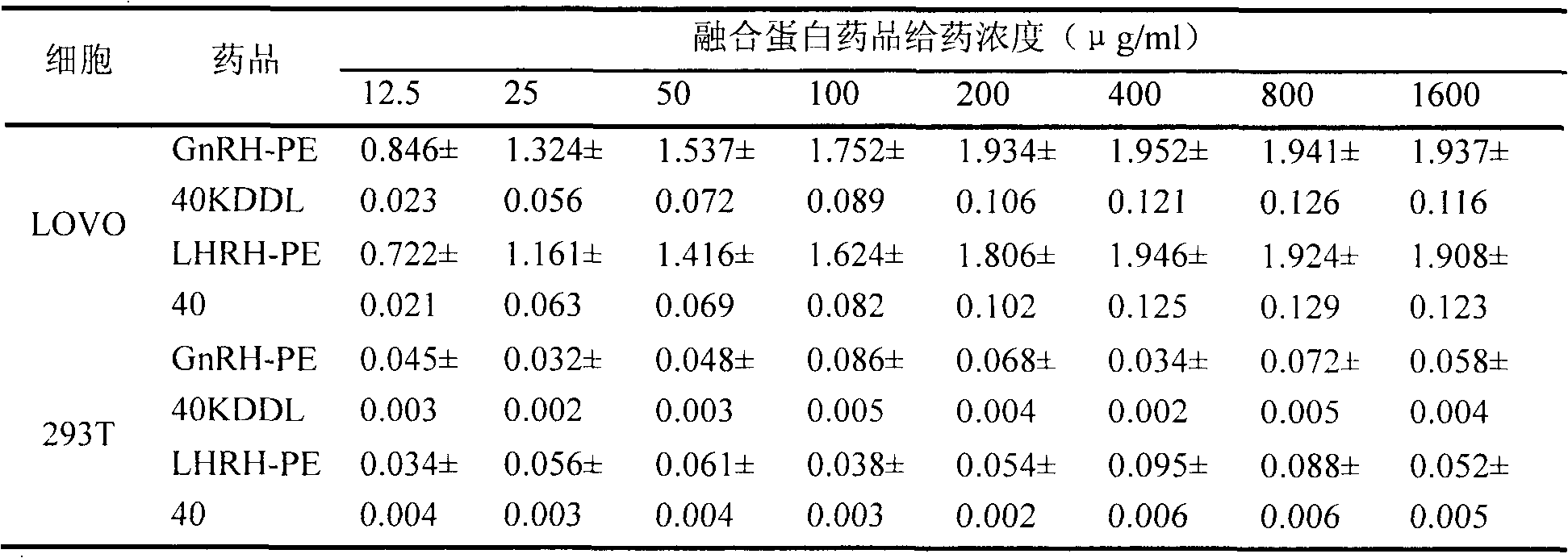
Novel gonadotropin releasing hormone oriented fusion protein mutant
A fusion protein and mutant technology, applied in the direction of peptide/protein components, hybrid peptides, drug combinations, etc., can solve the problems of invading normal cells, easy shedding of chemotherapeutic drugs, and drug failure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0022] Construction of Example 1 Recombinant Fusion Protein Particle and Acquisition of Engineering Bacteria
[0023] Using the designed amino acid sequence of SEQ ID NO1 as a template, according to the central rule, select the codon preferred by E. coli to obtain the corresponding nucleic acid sequence of the fusion protein, and then use the whole gene synthesis technology to obtain the above target gene fragment. The recombinant gene vector pUC-GnRH is obtained through the recombination technique known to those skilled in the art, and the sequence of the gene fragment inserted into the vector is determined. After verifying the correctness of its gene sequence, amplify and save, cut out the target gene fragment from the vector, insert it into the expression vector, and name the recombinant plasmid pGnRH. Then use CaCl 2 Transform Escherichia coli BL21 by using the method, spread on the surface of the screening plate medium containing antibiotics, select positive colonies and...
example 2
[0024] Example 2 Expression of the protein of interest
[0025] 1) Shake flask expression: the genetically engineered bacteria pGnRH / BL21 prepared as above was cultured overnight in LB medium in shake flask (37°C, 210rpm), then inoculated into LB medium at a volume ratio of 1:30, and incubated at 37°C After culturing for 3 hours, 0.1 mM IPTG was added for induction for 5 hours. The collected bacteria were analyzed by SDS-PAGE electrophoresis, and it was found that the fusion protein (43KD) was mainly expressed in soluble form, and the expression amount accounted for 38% of the total protein of the bacteria.
[0026] 2) Large-scale fermentation expression:
[0027] a. Medium composition:
[0028] a) Seed liquid medium (LB):
[0029] Tryptone: 10 g / L, yeast powder: 5 g / L, sodium chloride: 10 g / L.
[0030] b) Tank culture medium (15 liters):
[0031] Disodium hydrogen phosphate: 250 grams, potassium dihydrogen phosphate: 110 grams, sodium chloride: 13. grams, ammonium chlori...
example 3
[0039] Example three purpose protein purification:
[0040] (1) Hydrophobic chromatography
[0041] Use hydrophobic chromatography medium (Octyl Sepharose 4 Fast Flow), balance solution A with 25mM Tris-HCl, pH8, 1M ammonium sulfate, after equilibrium, load the centrifuged supernatant of the lysate (supplement ammonium sulfate to 1M), and balance with A After 5 column volumes, linear gradient elution, 0-20 column volumes, 1-0.1M ammonium sulfate, the fusion protein peak was collected.
[0042] (2) Anion exchange chromatography
[0043] Use Source 30Q anion exchange chromatography medium, balance solution A is 25mM Tris-HCl, pH8, balance, the sample obtained in the previous step is diluted 4 times with water, load, balance, linear gradient elution, 0-20 column volumes, 0- 0.5M NaCl, collect the target fusion protein peak.
[0044] (3) Anion exchange chromatography
[0045] Use Q Sepharose High Performance chromatography medium, the balance solution is 20mM PB, pH7.4, the sa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com