Alkali-resistant low-temperature alpha-galactosidase AgaAJB13 and genes thereof
A galactosidase, alkali-resistant technology, applied in the field of genetic engineering, to achieve the effect of wide action temperature range, wide application, good thermal stability and protease resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: α-galactosidase gene agaAJB13 clone
[0037] Extract the genomic DNA of Sphingomonas: centrifuge the 2-day cultured bacteria liquid to get the bacteria, add 1mL lysozyme, treat at 37°C for 60min, then add the lysate, lyse in a water bath at 70°C for 60min, and mix every 10min. Centrifuge at 10000 rpm for 5 min at 4°C. The supernatant was extracted in phenol / chloroform to remove impurity proteins, and then an equal volume of isopropanol was added to the supernatant. After standing at room temperature for 5 minutes, centrifuge at 10,000 rpm for 10 minutes at 4°C. The supernatant was discarded, the precipitate was washed twice with 70% ethanol, dried in vacuo, dissolved by adding an appropriate amount of TE, and stored at -20°C for later use.
[0038]According to the conserved sequence of the 36th family of glycoside hydrolases ([F / L / V]-[L / V]-[L / M / V]-D-D-G-W-F and E-P-E-M-[V / I]-[N / S]-[ P / E]) designed and synthesized degenerate primers GH36F and GH36R (Table ...
Embodiment 2
[0043] Example 2: Preparation of recombinant α-galactosidase
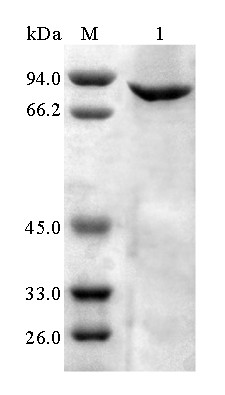
[0044] The expression vector pET-28a (+) was double digested ( Eco RI and Hind III), while the gene encoding α-galactosidase agaAJB13 double enzyme digestion ( Eco RI and Hind III), the α-galactosidase digested by the above enzyme agaAJB13 Connect with the expression vector pET-28a (+) to obtain the gene containing α-galactosidase agaAJB13 The recombinant plasmid pET- agaAJB13 And transform Escherichia coli BL21 (DE3) to obtain recombinant Escherichia coli strain BL21(DE3) / agaAJB13 .
[0045] Take the recombinant plasmid pET- agaAJB13 of E. coli BL21(DE3) strains and those containing only pET-28a(+) empty plasmid E. coli BL21 (DE3) strain was inoculated in LB (50 μg / mL Kan) culture medium with 0.1% inoculum, and shaken rapidly at 37°C for 16 hours. Then inoculate the activated bacterial solution into fresh LB (50 μg / mL Kan) culture solution with 1% inoculum, and culture with rapid shaki...
Embodiment 3
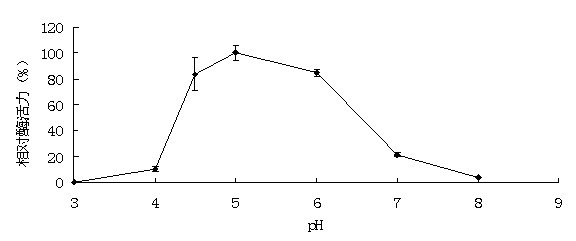
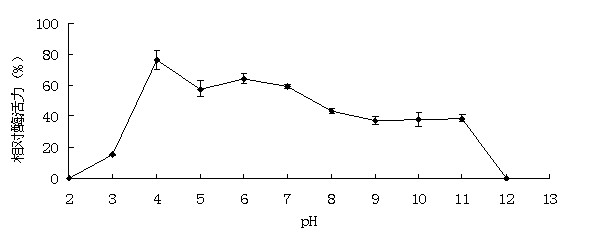
[0046] Example 3: Activity analysis of recombinant α-galactosidase
[0047] The enzyme activity assay method used p NPG method. Will p NPG was dissolved in 0.1 M buffer to make a final concentration of 2 mM. The reaction system contains 50 μL of appropriate enzyme solution and 450 μL of 2 mM substrate. After the substrate was preheated at the reaction temperature for 5 minutes, add the enzyme solution and react for 10 minutes, then add 1.5mL 1M Na 2 CO 3 The reaction was terminated, and after cooling to room temperature, the released p NP. 1 enzyme activity unit (U) is defined as decomposing per minute p NPG produced 1 μmol p Amount of enzyme required for NP. The method for determining the activity of the substrate raffinose, soybean meal and cotton meal is 3,5-dinitrosalicylic acid (DNS) method: the substrate is dissolved in 0.1M buffer to make the final concentration 0.5% (w / v); the reaction system contains 100 μL of appropriate enzyme solution and 900 μL of subst...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com