Glucagon-likepeptide1 derivatives and use thereof
A technology of drugs and compounds, applied in the direction of glucagon, hormone peptides, specific peptides, etc., can solve the problems of GLP-1 instability and limit the clinical application of GLP-1, achieve long plasma half-life, significant effect, hypoglycemic effect of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
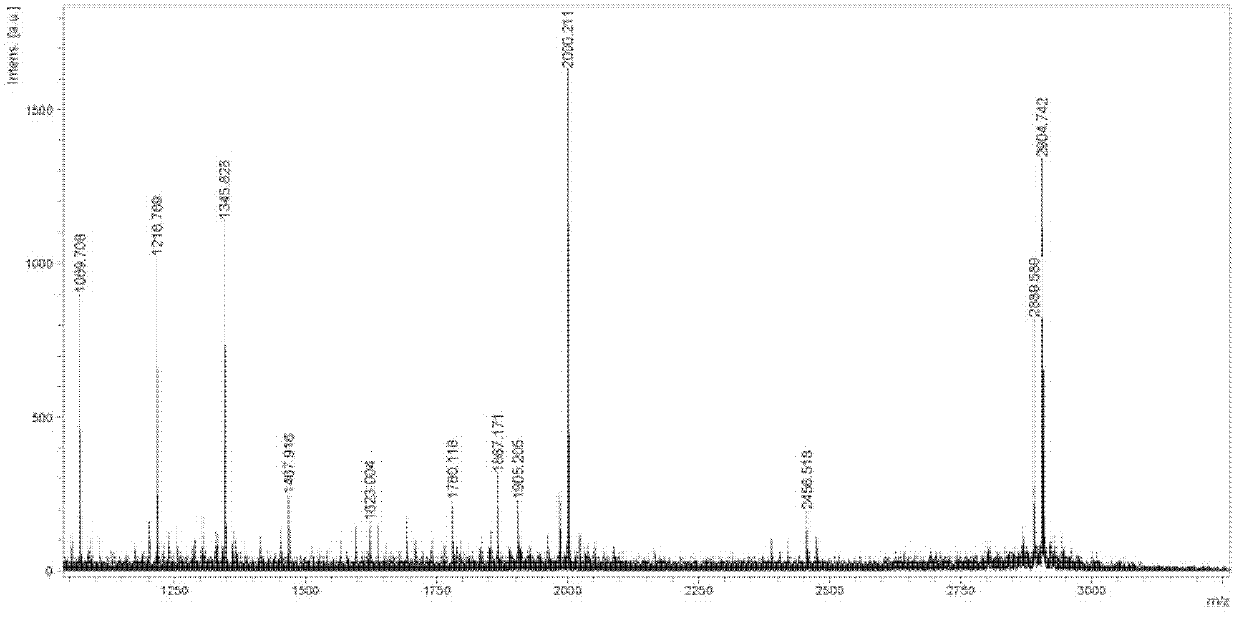
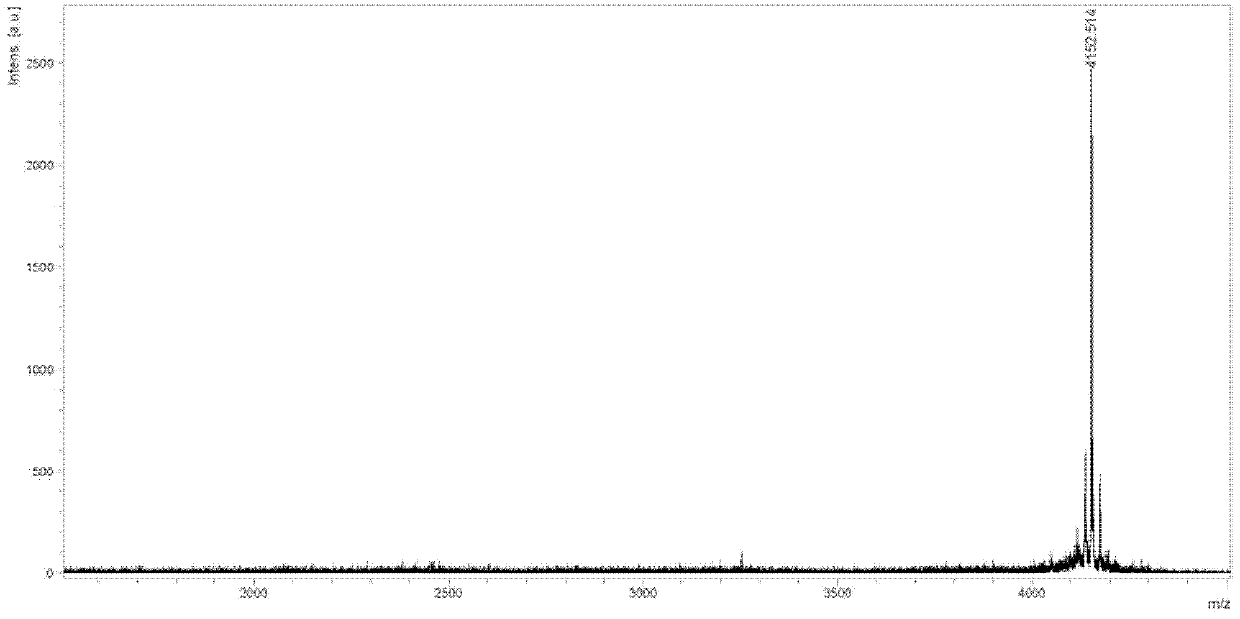
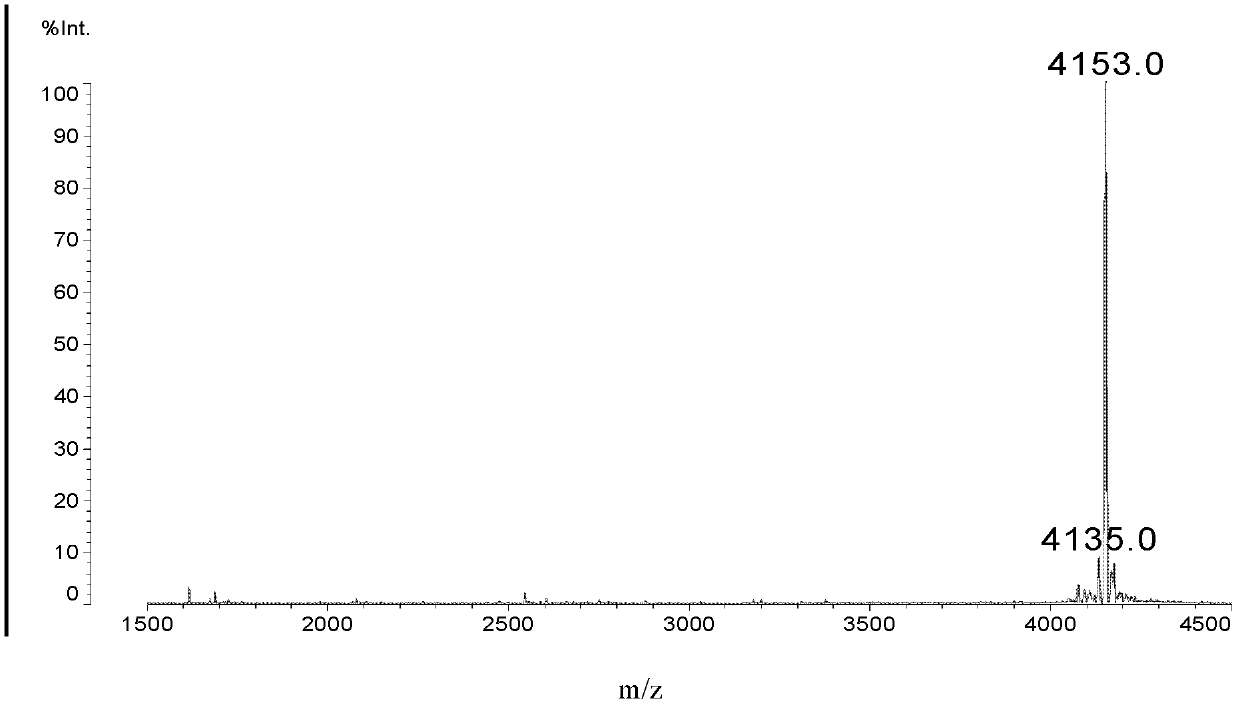
Image
Examples
Embodiment 1
[0031] I, synthetic intermediate (called Dimer)
[0032] The structure of Dimer is as follows:
[0033]
[0034] The specific synthesis method is as follows:
[0035] 1. Preparation of compound 01·HCl
[0036] Below -10°C, add 80ml of thionyl chloride dropwise to 250ml of methanol, complete the dropwise addition within 2 hours, and stir at room temperature for 1 hour. Add 40 g of L-alanine and stir overnight. Then, the temperature was raised to reflux for 4 hours. After cooling, the solvent was removed under reduced pressure to constant weight to obtain 80 g of crude compound 01·HCl.
[0037] 2. Preparation of Compound 02
[0038]40g of compound 01·HCl was dissolved in 350ml of DMF, 113g of benzyl bromide was added, and 150g of anhydrous potassium carbonate was added under stirring, and after stirring for 2 hours, the reaction was kept at 50°C for 2 hours. Then, the reaction solution was extracted with 1200ml of water and 400ml of ethyl acetate, separated, the ester l...
Embodiment 2
[0076] When synthesizing the main chain, replace Fmoc-Gly-Wang Resin with Rink Amide Resin, and the others are the same as in Example 1.
[0077] Among the above-mentioned Examples 1 and 2, those not indicating the reaction temperature all refer to normal temperature. Reagents A, B and C are solutions prepared by adding 80% phenol ethanol solution, redistilled pyridine and 5 g ninhydrin to 100 ml ethanol, respectively. Example 3 Determination of Glucose Tolerance
Embodiment 3
[0077] Among the above-mentioned Examples 1 and 2, those not indicating the reaction temperature all refer to normal temperature. Reagents A, B and C are solutions prepared by adding 80% phenol ethanol solution, redistilled pyridine and 5 g ninhydrin to 100 ml ethanol, respectively. Example 3 Determination of Glucose Tolerance
[0078] 1. Test group:
[0079] 90 ICR (Institute of Cancer Research) mice, all male, were divided into three batches according to body weight, 30 in each batch. Each batch of mice was fasted overnight and divided into two groups according to blood sugar: Vehicle group and compound group obtained in Example 1 (referred to as compound group). The vehicle group was only injected with physiological saline, and the compound group was injected with the compound obtained in Example 1 into the physiological saline.
[0080] 2. Test process:
[0081] The first batch of animals: mice were grouped according to blood sugar after fasting overnight, administered...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com