Quality detection method for RuanGanXiaoShui cataplasma
A quality inspection method and soft liver technology, applied in the digestive system, measuring devices, pharmaceutical formulas, etc., can solve the problems of qualitative and quantitative detection methods that do not reflect drug quality standards, cannot ensure drug efficacy, and cannot guarantee product quality, etc., to achieve Ensure efficacy and comprehensively reflect the effect of quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 10
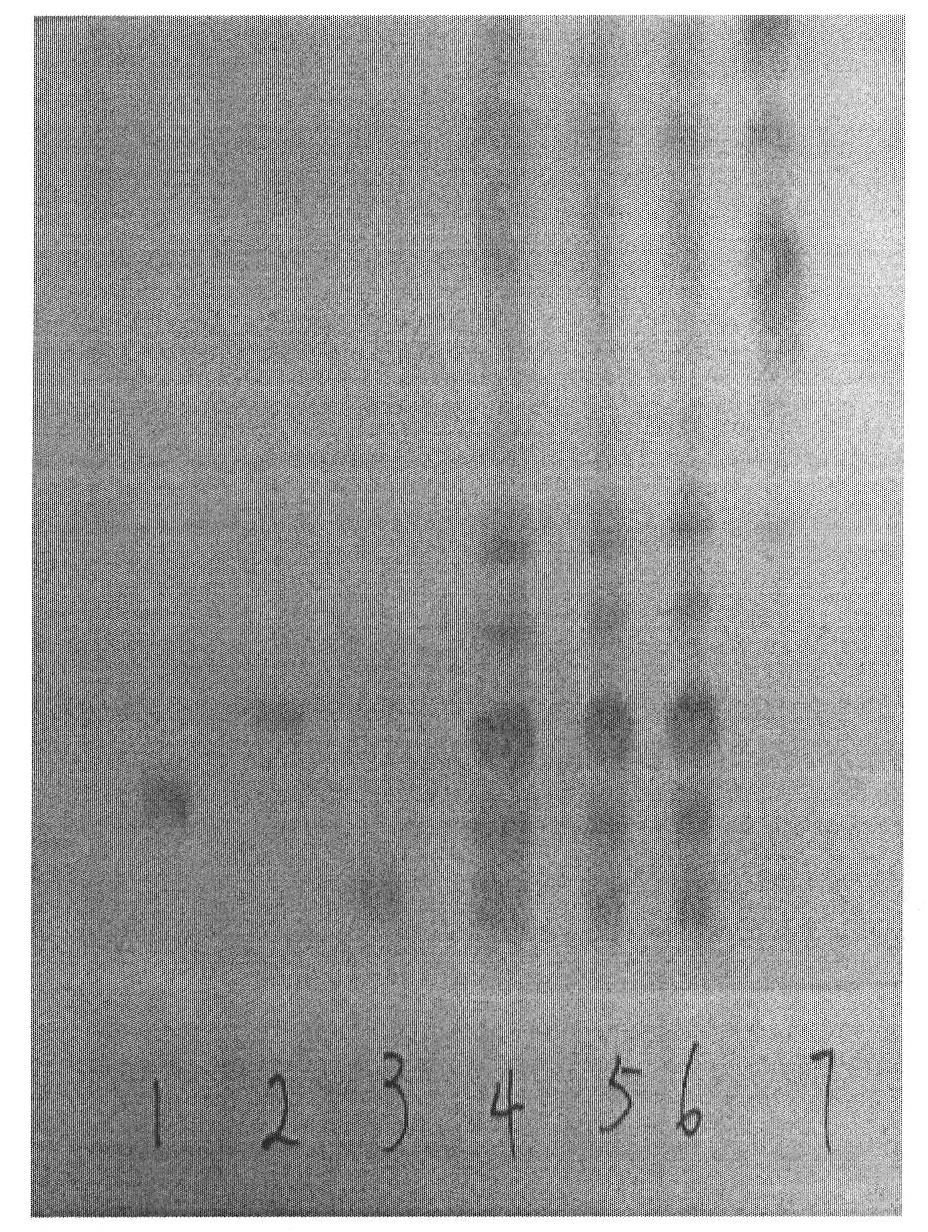
[0029] The TLC identification of embodiment 1 Panax notoginseng
[0030] 1) Preparation of the test solution:
[0031] Take 1 sticker of this product, cut it into pieces, add 50mL of water-saturated n-butanol, seal it, place it for 24h, treat it ultrasonically for 30min, take n-butanol solution, wash with 50mL of ammonia test solution, discard the ammonia solution, and wash with water 3 times , 30 mL each time, take the n-butanol layer and evaporate to dryness, add 1 mL of methanol to the residue to dissolve, transfer to a 10 mL volumetric flask, add methanol to the mark, shake well, and use it as the test solution;
[0032] 2) Preparation of reference substance solution:
[0033] Ginsenoside Rb 1 , Ginsenoside Rg 1 and notoginsenoside R 1 1mg each of the reference substances, mix, add 1mL of methanol, as the reference substance solution;
[0034] 3) Preparation of negative sample solution:
[0035] Take 1 paste of the negative sample lacking Panax notoginseng, and prepa...
Embodiment 2
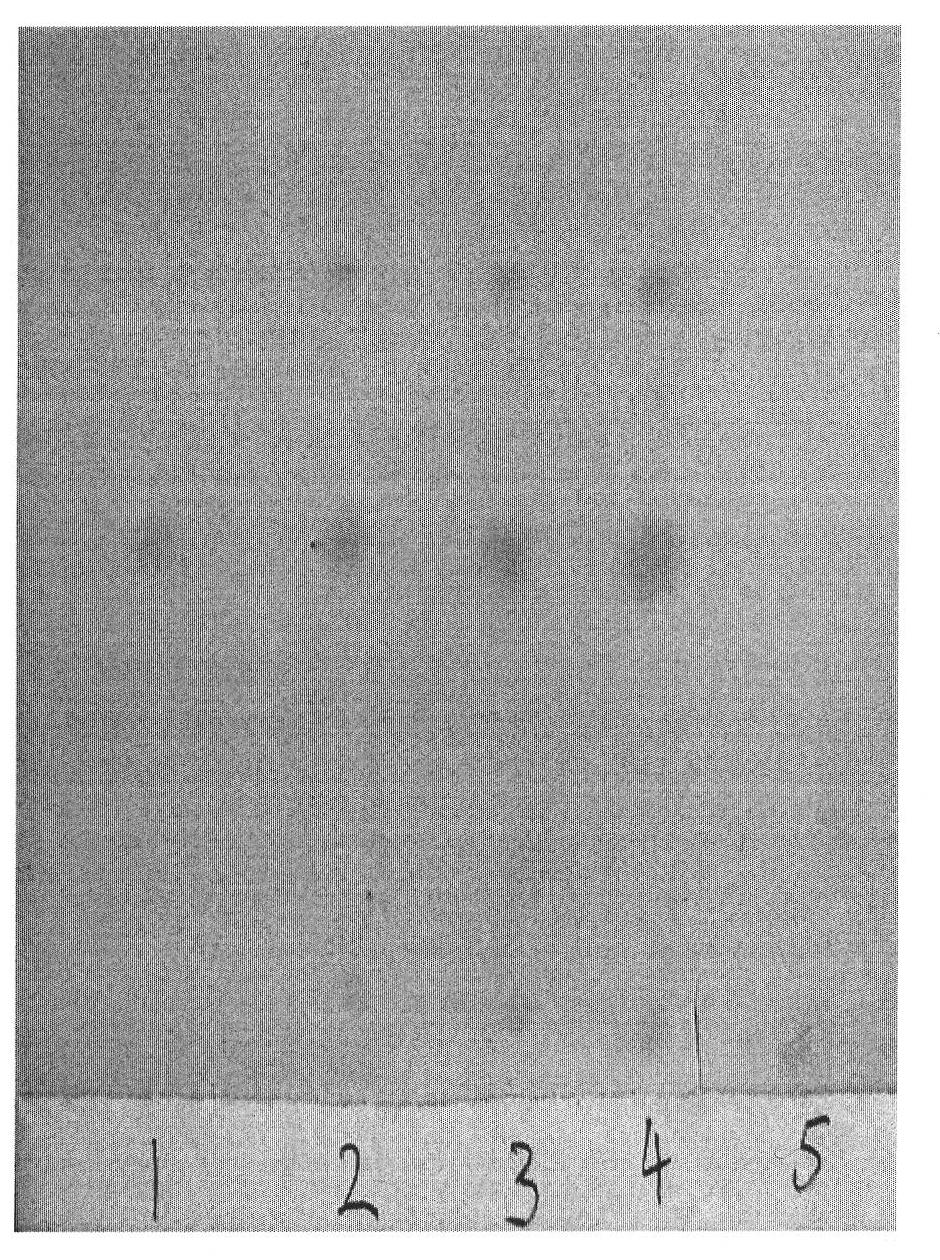
[0037] The TLC identification of embodiment 2 cinnamon
[0038] 1) Preparation of the test solution:
[0039] Take 1 paste of this product, cut it into pieces, add 50mL of ethanol, seal it, place it for 12h, ultrasonicate it for 30min, filter, and the filtrate is used as the test solution;
[0040] 2) Preparation of reference substance solution:
[0041] Get cinnamon aldehyde reference substance, add ethanol and make the solution that concentration is 2 μ g / mL, as reference substance solution;
[0042] 3) Preparation of negative sample solution:
[0043] Get 1 paste of the negative sample lacking cinnamon, prepare by the preparation method of need testing solution;
[0044] 4) According to the thin-layer chromatography test, draw 5 μL of each of the above three solutions, spot them on the same silica gel G thin-layer plate, use cyclohexane-ethyl acetate = 9:1 as the developing solvent, pre-saturate for 10 minutes, and develop upward , with a span of 8cm, take it out, dry i...
Embodiment 3
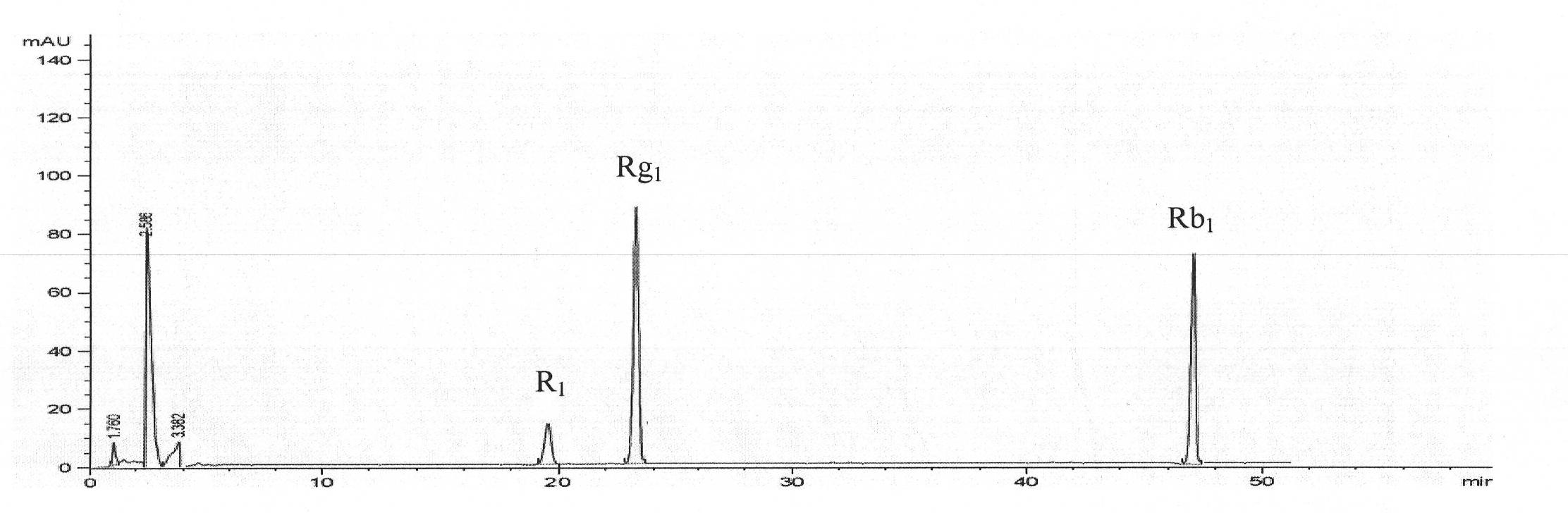
[0045] Embodiment 3 Assay
[0046] 1. Instruments and reagents
[0047] Agilent1100 high performance liquid chromatography, G1311A quaternary pump, G1322A degasser, G1315B DAD detector, HP chemstation chemical chromatography workstation; AL204 Mettler Toledo analytical balance (Switzerland); RC-3B drug dissolution apparatus (Tianjin University Radio Factory).
[0048] Acetonitrile (chromatographically pure Fisher Chemical), other reagents are analytically pure, double distilled water; microporous membrane (0.45μm); Rugan Xiaoshui cataplasm (homemade); ginsenoside Rg 1 Reference substance, ginsenoside Rb 1 Reference substance and notoginsenoside R 1 Reference substance (National Institute for the Control of Pharmaceutical and Biological Products).
[0049] 2. Chromatographic conditions
[0050] Chromatographic column: Kromasil ODS (250×4.6mm, 5μm); mobile phase: acetonitrile (A) and water (B), gradient elution was carried out according to the ratio in Table 1, and the flow ra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com