Drug composition for relieving cough and reducing phlegm
A composition and drug technology, applied in the field of medicine, can solve the problems of weak disinfection and antiseptic effect, achieve remarkable effect and increase safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
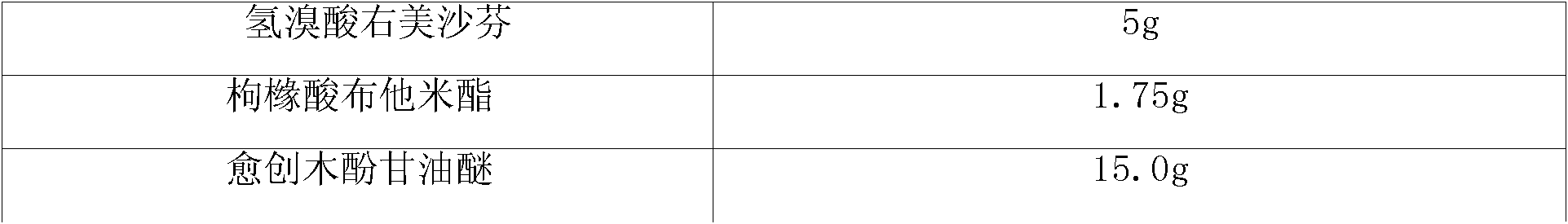
[0008] Embodiment 1: Compound butamidol granules:
[0009] prescription:
[0010]
[0011]
[0012] Process:
[0013] 1. Take dextromethorphan hydrobromide, butamidate citrate, and guaiacol glycerin and mix them evenly in equal increments.
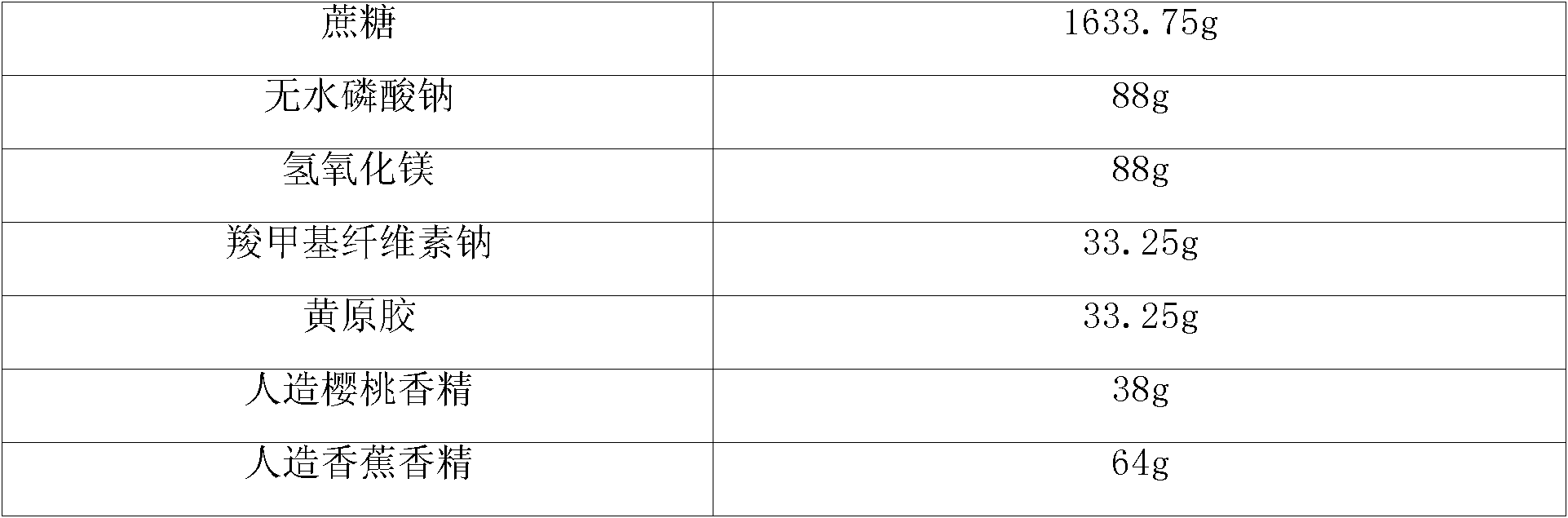
[0014] 2. Take the above-mentioned mixed medicine, anhydrous sodium phosphate, magnesium hydroxide, sodium carboxymethylcellulose, xanthan gum, and sucrose in sequence and mix them uniformly in equal increments, add purified water to make a soft material, and granulate with an 18-mesh sieve. Dry at 50°C and sieve with 18 meshes.
[0015] 3. Add artificial cherry flavor and artificial banana flavor, mix evenly, divide into packages, 2g per bag.
Embodiment 2
[0016] Embodiment 2: compound recipe butamidate tablet
[0017] prescription:
[0018] Dextromethorphan Hydrobromide
5g
butamidate citrate
1.75g
15.0g
80.0g
85.25g
10.0g
2.0g
1.0g
co-preparation
1000 pieces
[0019] Process:
[0020] 1. Take dextromethorphan hydrobromide, butamidate citrate, and guaiacol glycerin and mix them evenly in equal increments.
[0021] 2. Take the above-mentioned mixed medicine, lactose, microcrystalline cellulose, and croscarmellose sodium and mix them uniformly in equal increments, add purified water to make a soft material, granulate with a 18-mesh sieve, dry at 50°C, and dry for 24 Mesh sieve for whole grains.
[0022] 3. Add colloidal silicon dioxide and magnesium stearate, mix well, and press into tablets with a weight of 20...
Embodiment 3
[0023] Embodiment 3: compound butamidate capsules
[0024] prescription:
[0025] Dextromethorphan Hydrobromide
5g
butamidate citrate
1.75g
15.0g
80.0g
85.25g
10.0g
2.0g
1.0g
co-preparation
1000 capsules
[0026] Process:
[0027] 1. Take dextromethorphan hydrobromide, butamidate citrate, and guaiacol glycerin and mix them evenly in equal increments.
[0028] 2. Take the above-mentioned mixed medicine, lactose, microcrystalline cellulose, and croscarmellose sodium and mix them uniformly in equal increments, add purified water to make a soft material, granulate with a 18-mesh sieve, dry at 50°C, and dry for 24 Mesh sieve for whole grains.
[0029] 3. Add colloidal silicon dioxide and magnesium stearate, mix well, fill, and each capsule weighs 200mg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com