Method for producing recombinant human interleukin-21 by using Pichia pastoris
A technology for human interleukin and Pichia pastoris, which is applied in the field of bioengineering and can solve the problems of increased molecular weight and low expression level.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
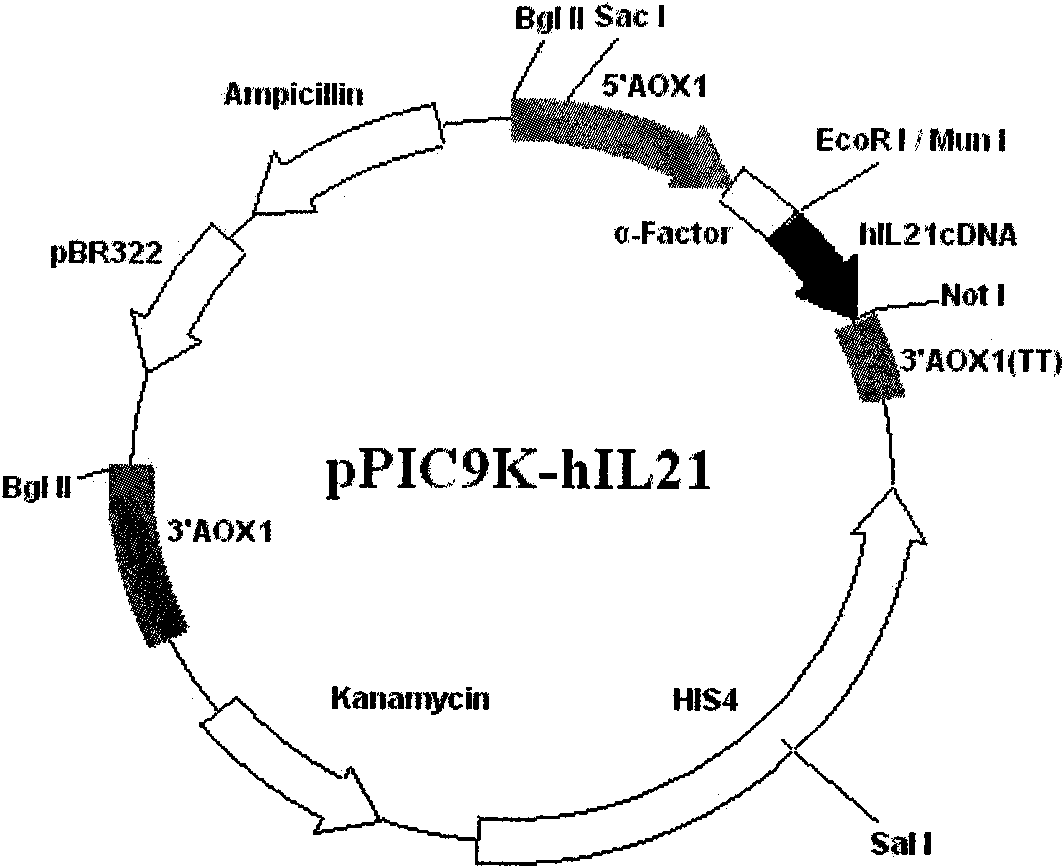
[0062] Construction of rhIL-21 engineering bacteria
[0063] 1. Acquisition and analysis of hIL-21 gene fragment sequence
[0064] First, the gene encoding hIL-21 was analyzed, and the results showed that the gene does not contain the sequence encoding the substrate of Pro-Glu-Ser-Thr, which activates proteolytic enzymes, nor does it contain the sequence encoding the gene that is susceptible to lysosomal cleavage. The sequence of X-Phe-X-Arg-Gln, Gln-Arg-X-Phe-X (X = any amino acid) of At the same time, considering that the codon preference of yeast is different from that of humans, the CAI value of hIL-21 coding gene expressed in Pichia pastoris is 0.74, which has the potential of encoding high-level expression protein (>0.6), and the CAI value is synonymous. A statistical measure of codon bias that can be used to predict protein expression levels. Therefore, the lymphocytes were directly isolated from the peripheral venous blood of healthy people, the total RNA of the cell...
Embodiment 2
[0081] Induced expression of hIL-21 in shake flasks
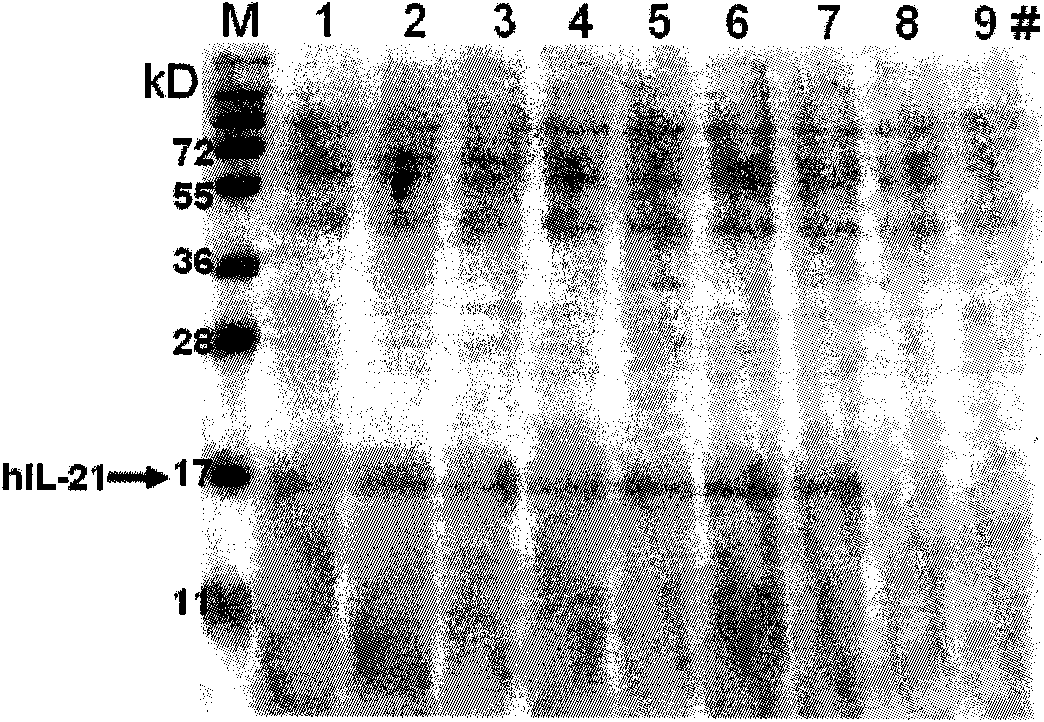
[0082] 1. Screen the engineering bacteria with the highest expression
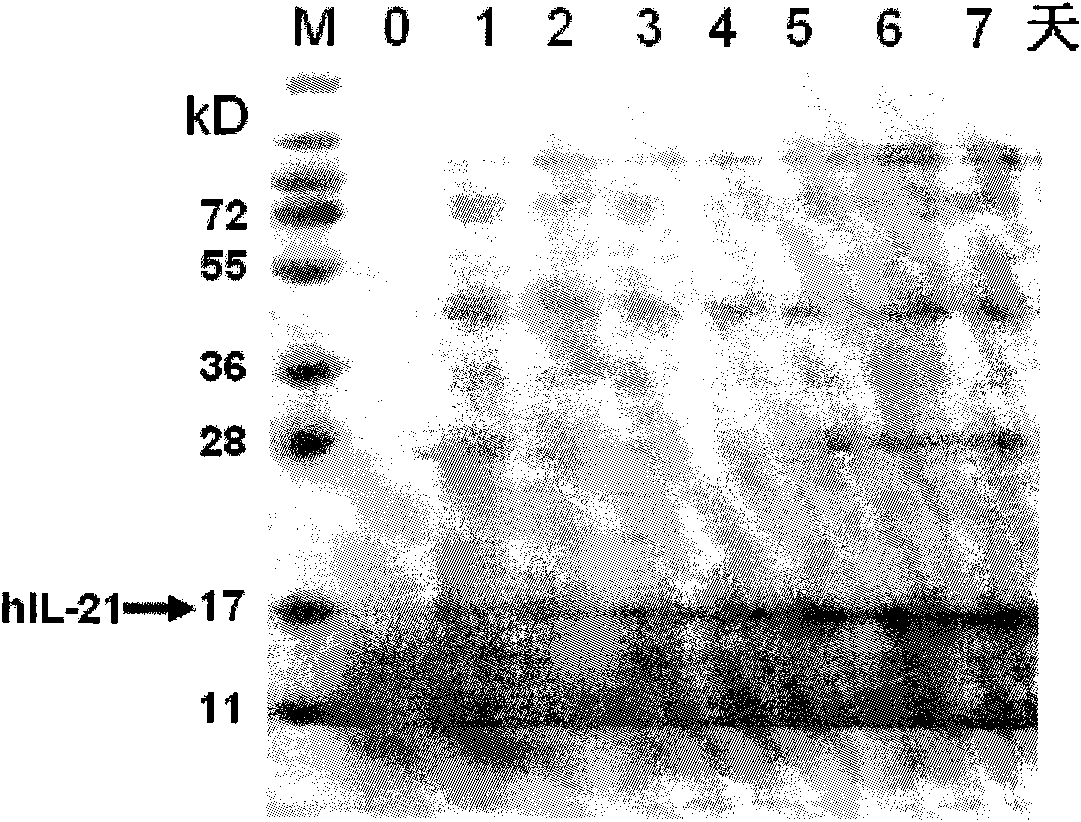
[0083] A two-step culture method was adopted, and the expression level of each transformant was expressed in shake flasks. The example was to pick multi-copy integrated recombinant transformants and inoculate them in 10mL BMGY medium, and culture them with shaking at 28-30°C and 250r / min until the bacteria When the OD600 of the solution is 2-6, centrifuge to remove the supernatant, replace it with BMMY medium to continue culturing, add methanol every 24 hours to a final concentration of 0.5%, and collect the bacterial solution after continuous induction for 72 hours. The supernatant was collected after the bacterial solution was centrifuged, part of the supernatant was concentrated with trichloroacetic acid, and the relative molecular weight of rhIL-21 was verified to be 16kD by 15% SDS-PAGE electrophoresis. Using IL-21 polyclonal antibody to do Wester...
Embodiment 3
[0091] Detection of hIL-21
[0092] Using a fast protein liquid chromatography system ( ), the protein in the expression supernatant was purified using the cation exchange medium SPSepharose Fast Flow. Use ABIPROCISE TM 492cLC (GC320078) instrument detects the first 15 amino acids of the N-terminal of purified rhIL-21. The detection method is based on the protein N-terminal sequencing standard method (SCI-S-006). The first 15 amino acids of the rhIL-21 N-terminal sequence are: Acid-Valine-Glutamic Acid-Leucine-Glutamine-Aspartic Acid-Arginine-Histidine-Methionine-Isoleucine-Arginine-Methionine- Arginine-glutamine-leucine (YVELQ DRHMI RMRQL) further confirmed that the expression product was rhIL-21.
[0093] In addition, the results of biological activity experiments show that the expression product can stimulate the proliferation of human lymphocytes, and the stimulation degree is the same as that of the commercially available IL-21 standard product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Relative molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com