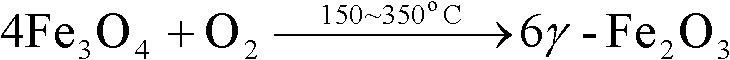Magnetic catalyst for denitration of NH3-SCR smoke and application thereof
A technology of NH3-SCR and magnetic materials, applied in physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, climate sustainability, etc., to achieve gas-solid contact reduction, good regulation and control characteristics, The effect of low raw material cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
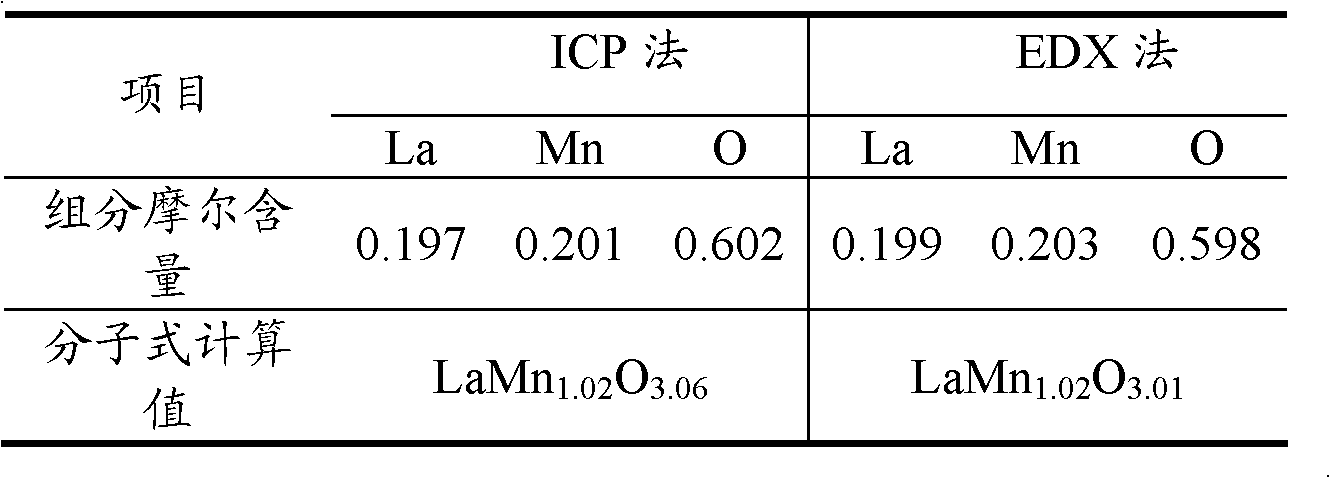
[0030] The nitrate of lanthanum and manganese is prepared according to the catalyst chemical formula LaMnO 3 The atomic ratio is made into an aqueous solution, and the mixed salt solution is added dropwise to the citric acid aqueous solution while stirring in a water bath at 30°C. The molar number of citric acid in the solution is equal to the molar number of lanthanum added. Continue to stir for 30 minutes after the dropwise addition, then concentrate the solution to gel, and then move it to a 110°C oven to dry for 12 hours to obtain a loose solid, then decompose it in the air at 500°C for 2 hours, and finally calcinate it at 800°C for 8 hours in an air atmosphere. After cooling, the desired catalyst carrier is obtained. Preliminary magnetic tests were carried out on the prepared catalyst carrier with a magnet, and it was found that the catalyst carrier was magnetic. ICP and EDX determine the elemental composition of the carrier as shown in Table 1, indicating that the carri...
Embodiment 2
[0037] The nitrate of lanthanum, potassium, manganese is prepared by the catalyst chemical formula La 0.8 K 0.2 MnO 3 The atomic ratio is made into an aqueous solution, and the mixed salt solution is added dropwise to the citric acid aqueous solution while stirring in a water bath at 30°C. The molar number of citric acid in the solution is equal to the molar number of lanthanum added. Continue to stir for 30 minutes after the dropwise addition, then concentrate the solution to gel, and then move it to a 120°C oven to dry for 12 hours to obtain a loose solid, then decompose it in the air at 500°C for 2 hours, and finally calcinate it at 900°C for 6 hours in an air atmosphere. After cooling, the desired catalyst carrier is obtained. Preliminary magnetic tests were carried out on the prepared catalyst carrier with a magnet, and it was found that the catalyst carrier was magnetic. ICP and EDX determine the elemental composition of the support as shown in Table 2, indicating tha...
Embodiment 3
[0044]The nitrate of lanthanum, copper, manganese is prepared according to the catalyst chemical formula LaCu 0.2 mn 0.8 o 3 The atomic ratio is made into an aqueous solution, and 28wt% ammonia water is used as a precipitating agent for dropwise addition, and the final pH value is controlled at about 10. After the precipitate was aged for 10 hours, it was suction-filtered, washed with deionized water, and washed 3 times with absolute ethanol, dried in an oven at 120°C for 12 hours, and the obtained sample was calcined at 800°C for 4 hours in an air atmosphere, and the required catalyst carrier was obtained after cooling . Preliminary magnetic tests were carried out on the prepared catalyst carrier with a magnet, and it was found that the catalyst carrier was magnetic. ICP and EDX determine the elemental composition of the support as shown in Table 3, indicating that the support structure is LaCu 0.2 mn 0.8 o 3 .
[0045] Table 3 Carrier ICP and EDX test report
[0046]...
PUM
| Property | Measurement | Unit |
|---|---|---|
| magnetic flux density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com