Eye drops and preparation method thereof
A technology of eye drops and solutions, which is applied in the fields of medical formulas, medical preparations containing active ingredients, cardiovascular system diseases, etc., can solve the problems of lack of anti-inflammatory and anti-lymphatic effects, confusion in the treatment of various corneal diseases, and problems affecting the eyes of patients. To prevent and treat corneal neovascularization, reduce the occurrence of complications and sequelae, and prevent and treat corneal neovascularization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
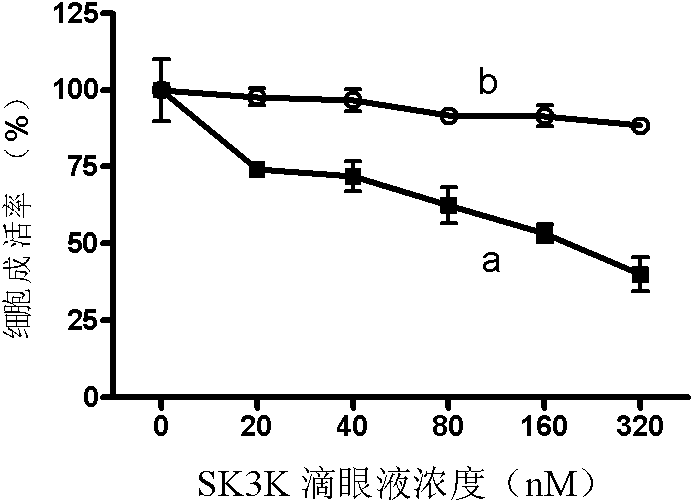
Image
Examples
Embodiment 1
[0042] Based on 10ml of eye drops, 7.73ml of PBS is used as the base solution, containing 200μl of fetal bovine serum, 100μl of low-molecular dextran, 200μl of HEPES, 100μl of tobramycin, 1.67ml of SA3K original solution, pH value of 7.2-7.4, osmotic pressure of 350~380mOsm / L.
[0043] Preparation method: Dissolve 1.67ml SA3K original solution and 100μg low-molecular-weight dextran in 2ml PBS solution, then mix with 200μl fetal bovine serum, 1MHERES 200μl and 10% tobramycin 100μl, add PBS solution to 10ml, adjust pH The value is 7.2-7.4, the osmotic pressure is 350-380mOsm / L, and it is sterilized by filtration through a 0.2μm membrane to obtain SA3K eye drops.
Embodiment 2
[0045] Based on 10ml of eye drops, 7.73ml of HBSS as the base solution, containing 200μl of fetal bovine serum, 100μl of low-molecular dextran, 200μl of HERES, 100μl of tobramycin, 1.67ml of SA3K original solution, pH value of 7.2-7.4, osmotic pressure It is 350~380mOsm / L.
[0046] Preparation method: Dissolve 1.67ml SA3K original solution and 100μg low-molecular-weight dextran in 2ml HBSS solution, then mix with 200μl fetal bovine serum, 1M HERES 200μl and 10% tobramycin 100μl, add HBSS solution to 10ml, adjust The pH value is 7.2-7.4, the osmotic pressure is 350-380mOsm / L, and it is sterilized by filtration through a 0.2μm membrane to obtain SA3K eye drops.
Embodiment 3
[0048] Based on 10ml of eye drops, 7.73ml of normal saline is used as the basic solution, containing 200ul of fetal bovine serum, 100μl of low-molecular dextran, 200μl of HERES, 100μl of tobramycin, 1.67ml of SA3K original solution, pH value of 7.2-7.4, osmotic The pressure is 350-380mOsm / L.
[0049] Preparation method: Dissolve 1.67ml SA3K original solution and 100μg low molecular weight dextran in 2ml normal saline solution, then mix with 200μl fetal bovine serum, 1M HERES 200μl and 10% tobramycin 100μl, add normal saline solution to 10ml , adjust the pH value to 7.2 to 7.4, the osmotic pressure to 350 to 380 mOsm / L, filter and sterilize through a 0.2 μm membrane to obtain SA3K eye drops.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com