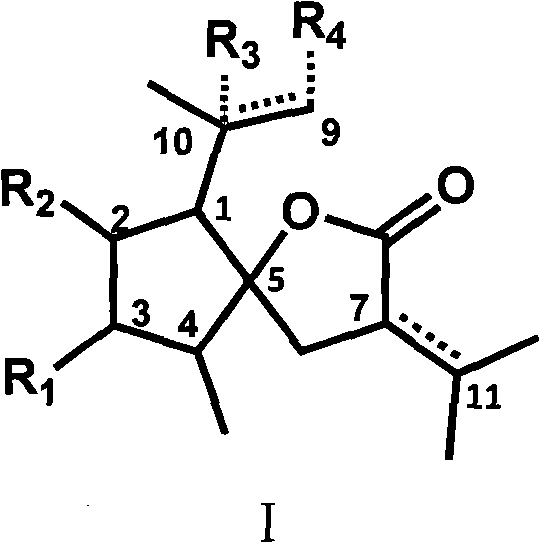
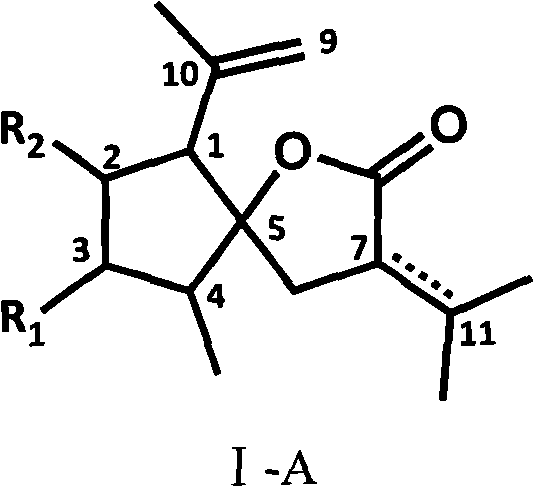
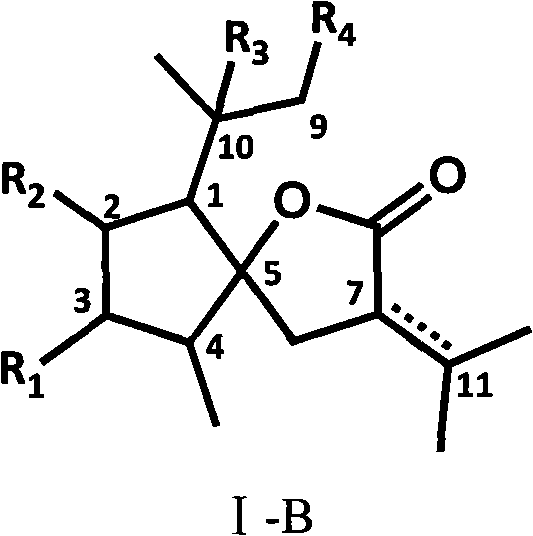
Curcumolide-like derivatives with antitumor activity and preparation method thereof
A technology of curcuma and lactone is applied in the field of curcumoid derivatives with anti-tumor activity and preparation thereof, and can solve the problems of low water solubility and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1: Preparation of 3α-hydroxycurcumolactone by biotransformation method:
[0059] 1: culture medium
[0060] A: Incline medium
[0061] Potato 20g, glucose 2g, agar 2g, distilled water 100mL.
[0062] B: liquid medium
[0063] Peptone 5g, glucose 15g, sucrose 15g, K 2 HPO 4 1.0g, KCl 0.5g, MgSO 4 0.5g, FeSO 4 ·7H 2 O0.01g, distilled water 1000mL, pH 7.0.
[0064] All the above media were steam sterilized at 115°C for 30 min.
[0065] 2: The process of biotransformation
[0066] Two-stage fermentation method (two-stage process):
[0067] A: The first stage of fermentation culture: inoculate the strain Aspergillus niger AS 3.739 on the slant of solid slant medium, culture at 28°C for 7 days, after producing abundant conidia, add 5mL sterile liquid medium to prepare spore suspension. The spore suspension was transferred into a 250 mL Erlenmeyer flask containing 50 mL of liquid medium, and cultured with shaking at 200 rpm at 28° C. for 72 hours to obtai...
Embodiment 2
[0073]Embodiment 2: the product of chemical transformation, then carry out biotransformation method to prepare 9,10-dihydroxylated curcumolactone:
[0074] Add 1% volumetric concentrated hydrochloric acid to 20 mL of chloroform, then add 20 mg of curcumadione, mix well and place at 20° C. for 24 hours to react. The solvent was removed by drying under reduced pressure to obtain a crude product, which was separated by column chromatography and eluted with a mixed solvent of n-hexane ethyl acetate, and concentrated under reduced pressure to obtain 15 mg of curcumolactone. By the biotransformation method described in Example 1, the product curcumolactone (dissolved in ethanol, sterilized through a sterile 0.22 μm filter) in step 3 is used as a substrate, with a final concentration of 0.1g / L, and then passed through the column Chromatography, eluting with a mixed solvent of n-hexane ethyl acetate, separated the converted product.
[0075] Structural identification of the transform...
Embodiment 3
[0077] Embodiment 3: the intermediate product of biotransformation, then carry out chemical transformation and prepare 3α-hydroxycurcumolactone:
[0078] 60 mg of curedione was converted by the biotransformation method in Example 1, and 18 mg of the intermediate product 3α-hydroxycuredione was isolated, which was added to 20 mL of chloroform containing 1% by volume concentrated hydrochloric acid, and reacted at 20° C. for 24 hours , dried under reduced pressure to remove the solvent, separated and purified by silica gel column chromatography to obtain white needle crystals. Structural identification of the product:
[0079] TOF-MS m / z: 275[M+Na] + , suggesting that the molecular weight is 252, consistent with the molecular weight of the substrate, and the molecular formula is C 15 h 24 o 3 . The NMR data were consistent with the NMR data of product 1, and the product 3 was identified as 3α-hydroxycurcumolactone.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com