Medicinal composition for treating ulcerative colitis and preparation method thereof
A drug and colon positioning technology, which is applied in drug combinations, pharmaceutical formulas, medical preparations with non-active ingredients, etc., can solve the problems of complex raw materials and components, and achieve the effects of convenient use, recurrence prevention, and improvement of diarrhea
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The preparation of embodiment 1 medicine of the present invention
[0038] 1. Preparation of Astragalus gastric drug-releasing unit:
[0039] Take 2.5g of astragaloside extract, 7.5g of astragalus polysaccharide extract, 18g of microcrystalline cellulose, 3.5g of micropowder silica gel, 2g of sodium carboxymethyl starch, powdered and mixed evenly through No. 6 sieve (100 mesh, 150 μm) A soft material is prepared with an appropriate amount of 50% ethanol as a wetting agent, extruded and spheronized to obtain pellets. Adopt Eudragit E100 alcohol-soluble coating, and its coating prescription is Eudragit E100 10g; Talcum powder 5g; PEG6000 1g; Water 2g; Ethanol 140g. Dissolve Eudragit E100 in about 70g of ethanol; dissolve PEG6000 in water; add talc powder to the remaining ethanol and homogenize at high speed for 20 minutes; add PEG6000 solution and talc powder suspension into Eudragit E100 solution, stir well to obtain the coating liquid. Adopt the fluidized bed coating t...
Embodiment 2
[0050] The preparation of embodiment 2 medicines of the present invention
[0051] 1. Prescription of gastric release unit preparation
[0052] Astragalus coated pellets
[0053] Astragalus polysaccharide extract 398mg (containing polysaccharide 355mg) Astragalus saponin extract 136mg (containing saponin 100mg)
[0054] Microcrystalline cellulose 961mg Micropowder silica gel 187mg
[0055] Carboxymethyl Starch Sodium 107mg Coating 90mg
[0056] Total 1879mg
[0057] 2. Prescription of colon-localized drug release unit preparation
[0058] 2.1pH-sensitive single-layer coated pellets
[0059] Matrine Extract 51mg (Matrine 50mg) Oxymatrine Extract 153mg (Oxymatrine 150mg)
[0060] Microcrystalline Cellulose 755mg Sodium Carboxymethyl Starch 61mg
[0061] Coating 306mg
[0062] Total 1326mg
[0063] 2.2 pH-sensitive double-layer coated pellets
[0064] Matrine Extract 51mg Oxymatrine Extract 153mg
[0065] Microcrystalline Cellulose 755mg Sodium Carboxymethyl...
Embodiment 3
[0074] Embodiment 3 The screening test of pharmaceutical adjuvant of the present invention and preparation technology
[0075] One, the preparation of drug stomach-colon release capsule of the present invention
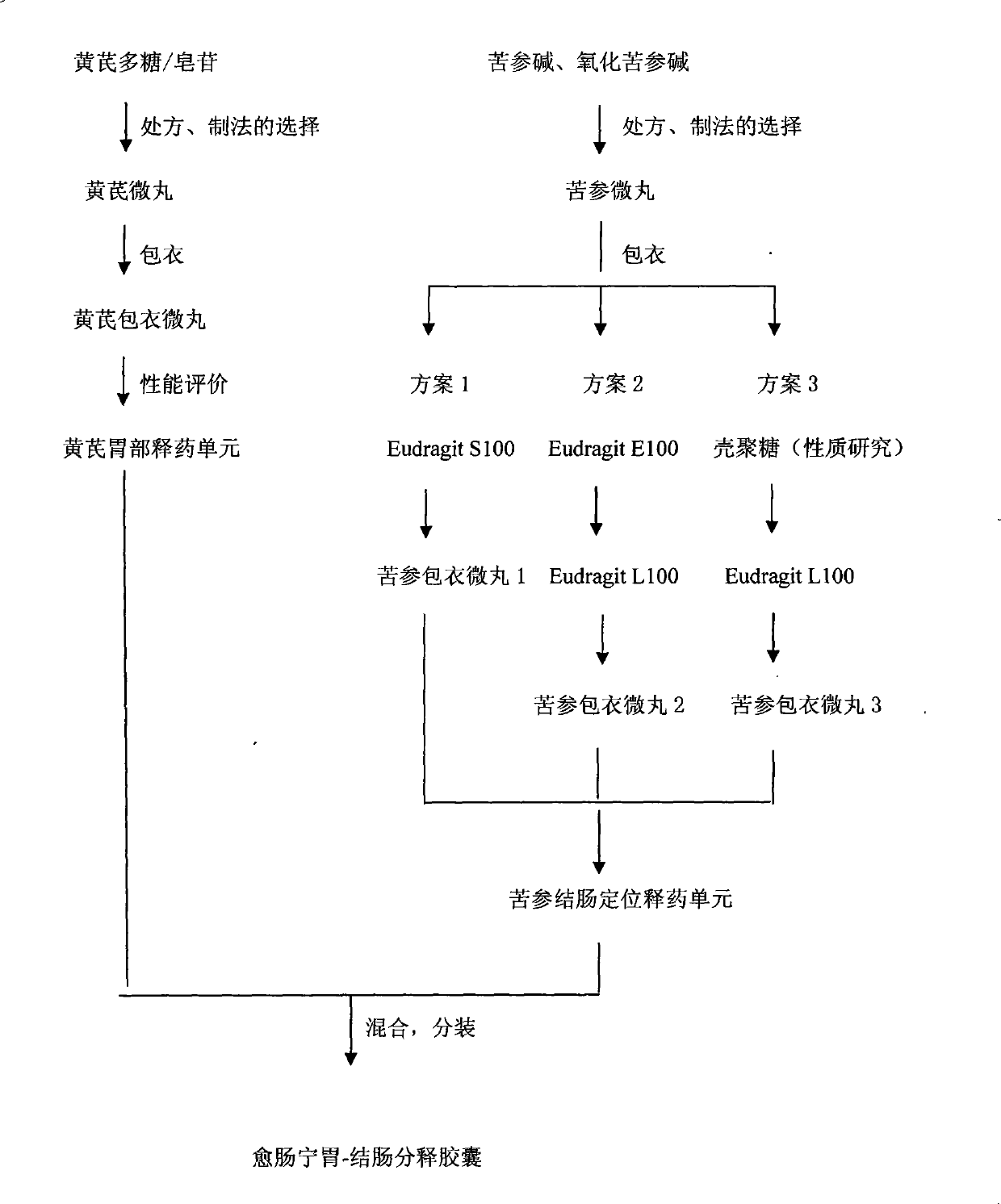
[0076] The medicine of the present invention is divided into two parts, wherein astragaloside and polysaccharide are prepared into preparation units capable of rapid drug release in the stomach, matrine and oxymatrine are prepared into preparation units with colon-localized drug release performance, and then The two preparation units are mixed and assembled into a compound drug delivery system including two kinds of drug release behaviors.
[0077] See the specific process route figure 1 .
[0078] 1 Instruments and reagents:
[0079] 1.1 Instrument
[0080] Spherical pellet granulator (Institute of Chemical Machinery, East China University of Science and Technology)
[0081] Friability monitor (FT-2000, Tianjin Sixin Technology Co., Ltd.)
[0082] Powder flowab...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
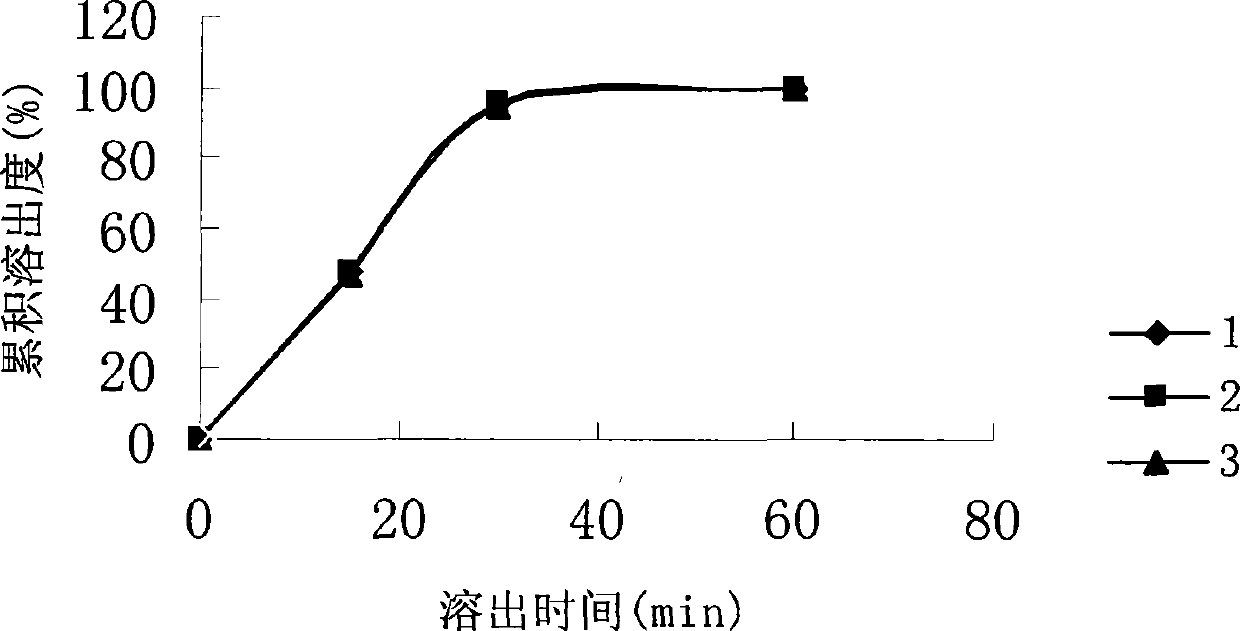
| cumulative release rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com