Method for preparing hydrotalcite
A technology of hydrotalcite and ammonia water, applied in chemical instruments and methods, oxide/hydroxide preparation, chromium oxide/hydrate, etc., can solve problems such as troubles, achieve good application prospects, simplify the process, and be cheap
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Aqueous ammonia and ammonium chloride were used to prepare 500ml of buffer solution with pH=10. Take 0.2mol AlCl 3 ·6H 2 O and 0.6mol MgCl 2 ·6H 2 O, add 200ml of water to form a mixed salt solution, slowly drop the mixed salt solution into the buffer solution under stirring at room temperature, continue stirring for 1 hour after the titration is completed, crystallize in an oven at 65°C for 20 hours, filter, and wash the filter cake Until the filtrate is neutral, it is dried at 55°C for 24 hours and then ground into a white powder to obtain chloride ion pillared magnesium aluminum hydrotalcite (Mg 0.75 Al 0.25 (OH) 2 Cl 0.25 mH 2 O), denoted as Mg 3 A1-Cl-LDHs.
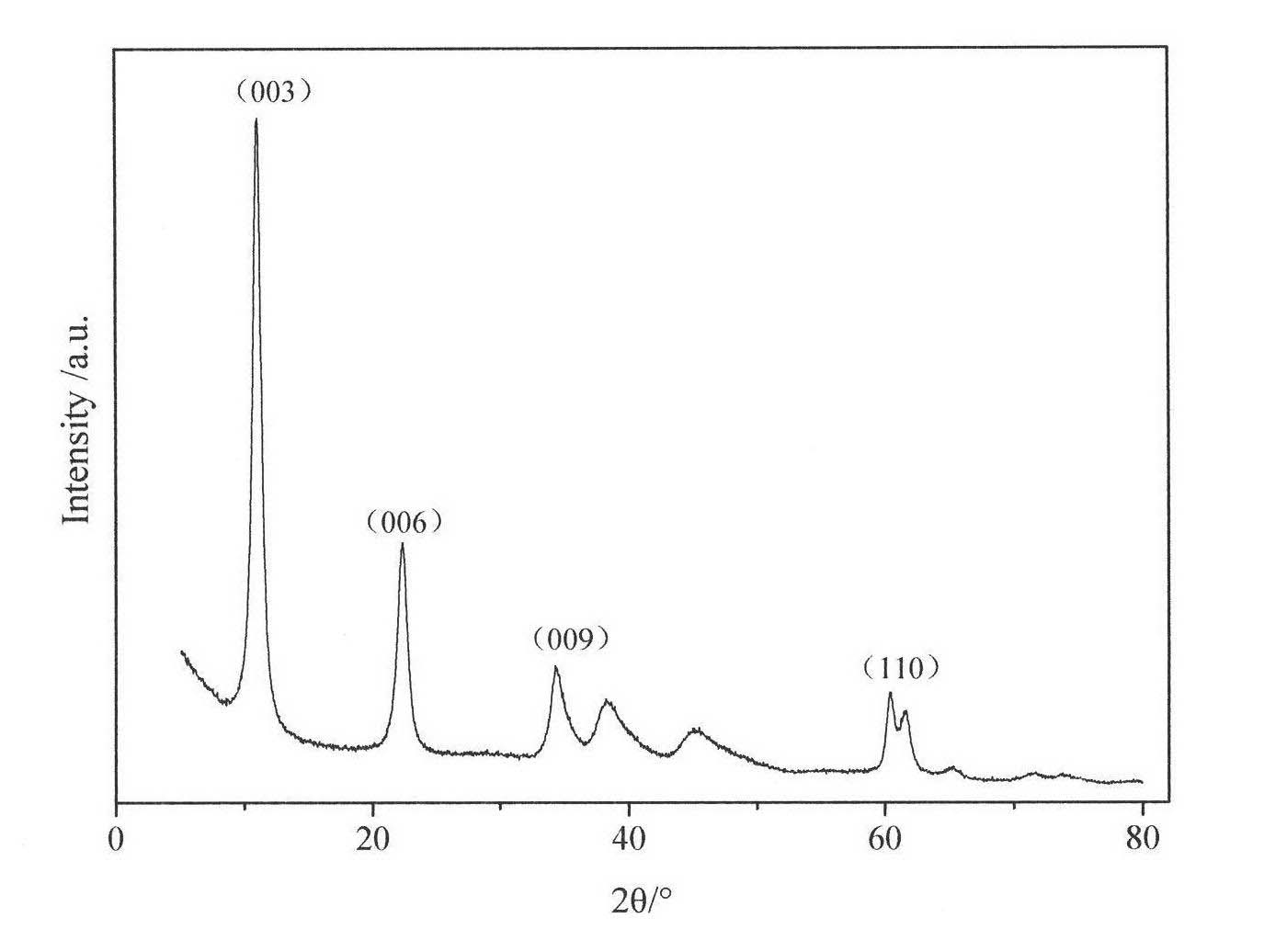
[0028] The synthesized Mg 3 Al-Cl-LDHs samples were analyzed by powder X-ray diffraction (PXRD), using ARL SCINTAG X-ray diffractometer, Cu Kα (0.1545nm), tube pressure 45kV, tube flow 40mA, scan speed 2° min -1 , 2θ ranges from 1.5 to 80°. Synthetic sample Mg 3 The XRD patterns of Al-Cl-LDHs are ...
Embodiment 2
[0031] Aqueous ammonia and ammonium chloride were used to prepare 500 ml of buffer solution with pH=9. Take 0.2mol AlCl 3 ·6H 2 O and 0.4mol MgCl 2 ·6H 2 O, add 200ml of water to make a mixed salt solution, slowly drop the mixed salt solution into the buffer solution under stirring at room temperature, continue stirring for 1.5h after the titration is completed, crystallize in an oven at 65°C for 24h, filter, and filter cake Wash until the filtrate is neutral, dry at 65°C for 18 hours, and then grind it into a white powder to obtain chloride ion pillared magnesium aluminum hydrotalcite (Mg 0.67 Al 0.33 (OH) 2 Cl 0.33 mH 2 O). XRD and SEM characterizations showed that the synthesized sample had a single crystal phase, a hexagonal plate shape, uniform grain size distribution, and an average particle size of about 90nm.
Embodiment 3
[0033] Aqueous ammonia and ammonium chloride were used to prepare 500ml of buffer solution with pH=10. Take 0.2mol AlCl 3 ·6H 2 O and 0.8mol MgCl 2 ·6H 2 O, add 200ml of water to make a mixed salt solution, slowly drop the mixed salt solution into the buffer solution under stirring at room temperature, continue stirring for 1.25h after the titration is completed, crystallize in an oven at 95°C for 6h, filter, and filter cake Wash until the filtrate is neutral, add ammonia water to the filtrate, adjust its pH to 10, and use it as a new buffer solution. The filter cake was dried at 55°C for 20 hours and then ground into a white powder to obtain chloride ion pillared magnesium aluminum hydrotalcite (Mg 0.8 Al 0.2 (OH) 2 Cl 0.2 mH 2 O). XRD and SEM characterizations showed that the synthesized sample had a single crystal phase, a hexagonal plate shape, uniform grain size distribution, and an average particle size of about 85nm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com