Application of cysteamine in preparing medicine for treating cancer
A technology of cysteamine and chemotherapeutic drugs, applied in the application field of cysteamine in the preparation of drugs for treating cancer, can solve problems such as cardiac conduction disorder, peripheral neuritis, treatment interruption, cardiotoxicity, etc. Effects with low side effects and biological toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1. Cysteamine can induce autophagy in HeLA cancer cells [Induce autophagy in human cervical cancer HeLa cells stably expressing LC3-eGFP, and use the autophagy promoter rapmycin as a positive control]
[0036] experimental method:
[0037] 1. The method for screening human cervical cancer cells stably expressing LC3-eGFP (LC3-eGFP / HeLa) is as follows:
[0038] (1) Human cervical cancer cell HeLa cells (purchased from Shanghai Institute of Cells, Chinese Academy of Sciences) were inoculated in 24-well cell culture plates (GIBCO, USA) with DMEM medium at a density of about 3-5×10 4 / well, at 37°C, 5% (volume percent) CO 2 Incubate overnight in an incubator until use.
[0039](2) Lipofectamine2000, 1.5 μl per well, was dissolved in 100 μl of serum-free and antibiotic-free DMEM medium, mixed well and incubated at room temperature for 5 min, and the plasmid LC3-eGFP (Invitrogen Company, USA) was 2 μg per well, dissolved in 100 μl of serum-free DMEM In the antibiot...
Embodiment 2
[0046] Example 2: Cysteamine induces autophagy in 293A cells [Induces autophagy in human embryonic kidney cells 293A stably expressing LC3-eGFP, using the autophagy promoter rapmycin as a positive control]
[0047] experimental method:
[0048] 1. The method for screening human embryonic kidney cells stably expressing LC3-eGFP (LC3-eGFP / 293A) is as follows:
[0049] (1) 293A human embryonic kidney cells (purchased from Shanghai Institute of Cells, Chinese Academy of Sciences) were inoculated in 24-well cell culture plates (GIBCO, USA) with DMEM medium at a density of about 3-5×10 4 / well, at 37°C, 5% (volume percent) CO 2 Incubate overnight in an incubator until use.
[0050] (2) Prepare the transfection complex, the method is as follows: 1.5 μl of Lipofectamine2000 per well, dissolved in 100 μl of DMEM (serum-free and antibiotic-free) medium, mixed well and incubated at room temperature for 5 minutes, 2 μg of LC3-eGFP per well, dissolved in 100 μl In DMEM (serum-free and a...
Embodiment 5
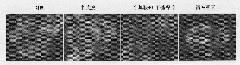
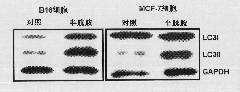
[0060] Example 5: Dose effect of cysteamine on autophagy in HeLa cells [transformation of endogenous protein LC3I to autophagy marker protein LC3II]
[0061] experimental method:
[0062] 1. HeLa cells were inoculated in DMEM medium (GIBCO, USA) 24-well cell culture plate, and the cell density of the 24-well plate was about 3-5×10 4 / well, at 37°C, 5% (volume percent) CO 2 Incubate overnight in an incubator until use.
[0063] 2. Add cysteamine with a final concentration of 0.5mM, 1.0mM, 1.5mM or 2.0mM to each well, and the cells without any treatment are the control group ( Figure 5 In the control), the final concentration of the autophagy promoter was 200nM rapamycin treatment (Sigma, USA) as a positive control for causing autophagy ( Figure 5 Rapamycin in), at 37°C, 5% (volume percentage) CO 2 In an incubator, the cells were collected after 24 hours of cell culture, and the cells were lysed with a cell lysate (purchased from Beyuntian Company), and then electrophoresi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com