Long-circulating ligustrazine composite injection and preparation method thereof
A technology of ligustrazine and composition, applied in the field of composition injection and preparation thereof, can solve the problems of ligustrazine drug treatment effect that is not very satisfactory to patients, low bioavailability, frequent administration and the like, so as to prolong the action time of the drug , Increase targeting function, reduce lipid deposition effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
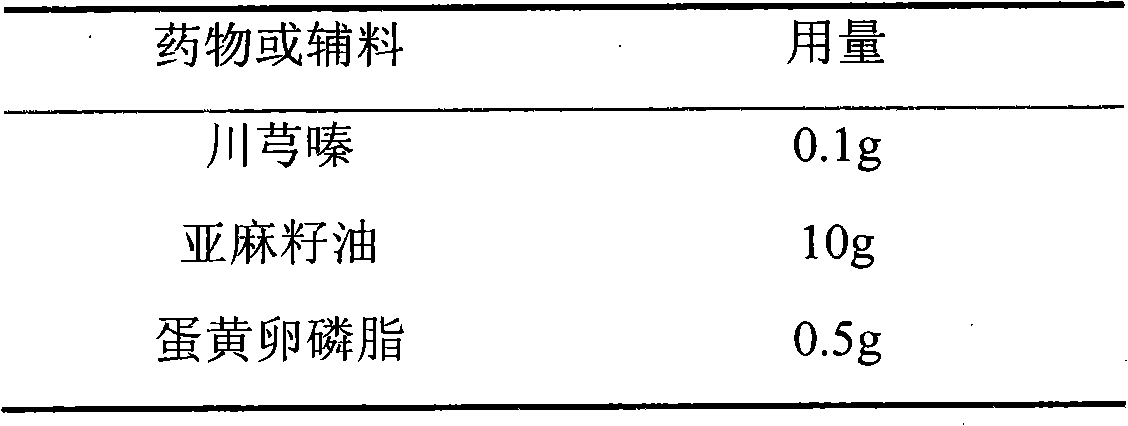
[0037] Example 1: 0.1% long-circulation ligustrazine composition injection preparation
[0038] prescription:
[0039]
[0040] method:
[0041] Take 10g of linseed oil, add 0.5g egg yolk lecithin, 0.1g polyethylene glycol-phospholipid (PEG 2000 -DSPE), under the protection of nitrogen, heat to 75°C, stir for about 10min to fully dissolve the added lipid compounds, then add 0.1g ligustrazine, 1g vitamin E and 0.03g selenium, dissolve and mix. Take another 80ml of water for injection, add 1g of glycerol, 0.5g of fructose, and 0.01g of EDTA. Under the protection of nitrogen, the oil solution containing ligustrazine is added to the glycerin aqueous solution under shearing and stirring conditions to prepare colostrum, and the total amount is adjusted to 100ml. The high-pressure homogenizer homogenizes 5-8 times, the homogenization pressure is about 100MPa, to the particle size range of 150nm-200nm, adjusts the pH 7.0-8.0, filters, divides, and seals. Rotate sterilize at 115°C for 30mi...
Embodiment 2
[0042] Example 2: 4% long-circulation ligustrazine composition injection preparation
[0043] prescription:
[0044]
[0045] method:
[0046] Take 10g of linseed oil, add 0.5g egg yolk lecithin, 0.1g polyethylene glycol-phospholipid (PEG 2000 -DSPE), under the protection of nitrogen, heat to 75°C, stir for about 10min to fully dissolve the added lipid compounds, then add 4g ligustrazine, 1g vitamin E and 0.03g selenium, dissolve and mix. Take another 80ml of water for injection, add 1g of glycerol, 0.5g of fructose, and 0.01g of EDTA. Under the protection of nitrogen, the oil solution containing ligustrazine is added to the glycerin aqueous solution under shearing and stirring conditions to prepare colostrum, and the total amount is adjusted to 100ml. The high-pressure homogenizer homogenizes 5-8 times, the homogenization pressure is about 100MPa, to the particle size range of 150nm-200nm, adjusts the pH 7.0-8.0, filters, divides, and seals. Rotate sterilize at 115°C for 30min. A...
Embodiment 3
[0047] Example 3: 0.1% long-circulating fat-soluble ligustrazine derivative composition injection preparation
[0048] prescription:
[0049]
[0050]
[0051] method:
[0052] Take 10g of linseed oil, add 0.5g egg yolk lecithin, 0.1g polyethylene glycol-phospholipid (PEG 2000 -DSPE), under the protection of nitrogen, heat to 75℃, stir for about 10min to fully dissolve the added lipid compounds, then add 0.1g of fat-soluble ligustrazine derivatives, 1g of vitamin E and 0.03g of selenium to dissolve Mix well. Take another 80ml of water for injection, add 1g of glycerol, 0.5g of fructose, and 0.01g of EDTA. Under the protection of nitrogen, the oil solution containing ligustrazine is added to the glycerin aqueous solution under shearing and stirring conditions to prepare colostrum, and the total amount is adjusted to 100ml. The high-pressure homogenizer homogenizes 5-8 times, the homogenization pressure is about 100MPa, to the particle size range of 150nm-200nm, adjusts the pH 7.0-8...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com