Recombinant human arginase fusion protein and application thereof
A technology of fusion protein and arginase, applied in the field of genetic engineering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
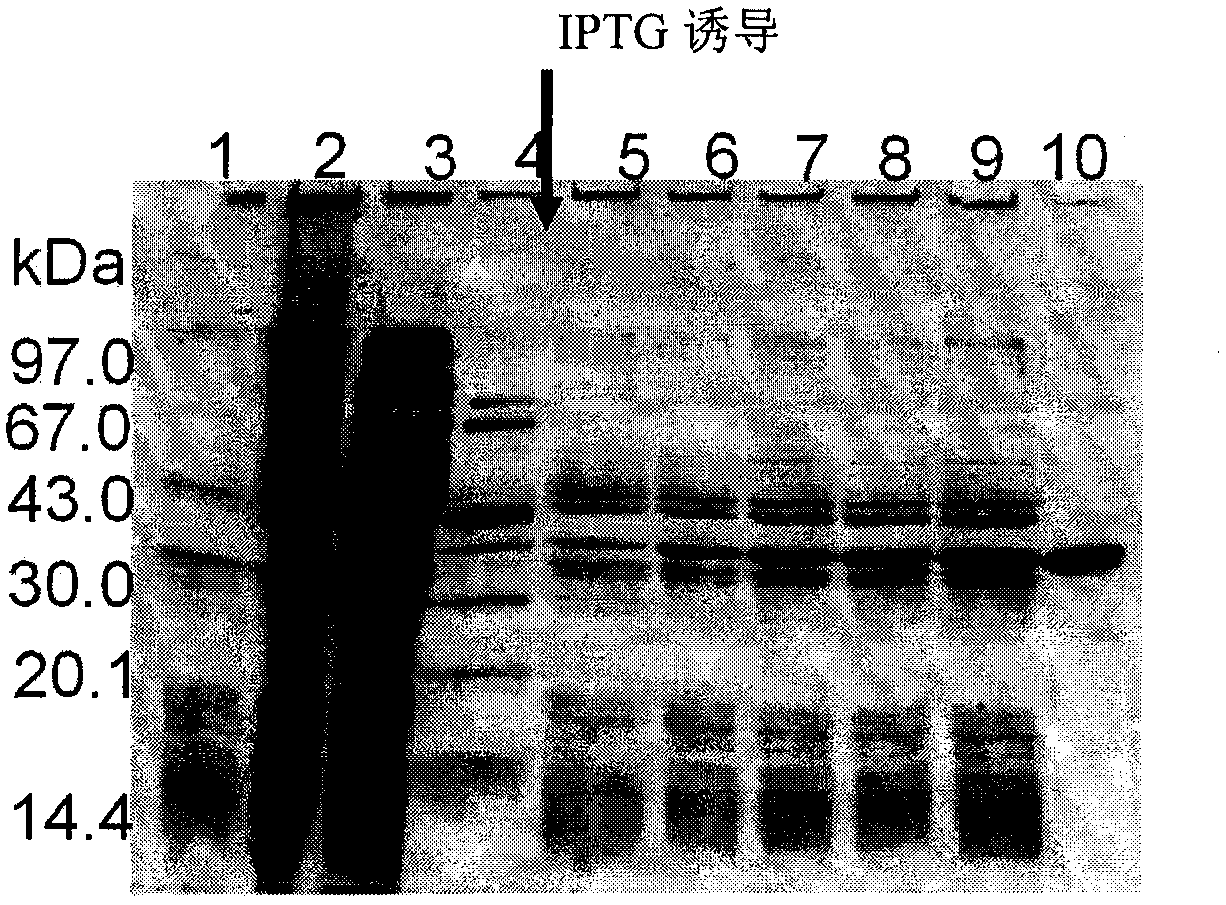
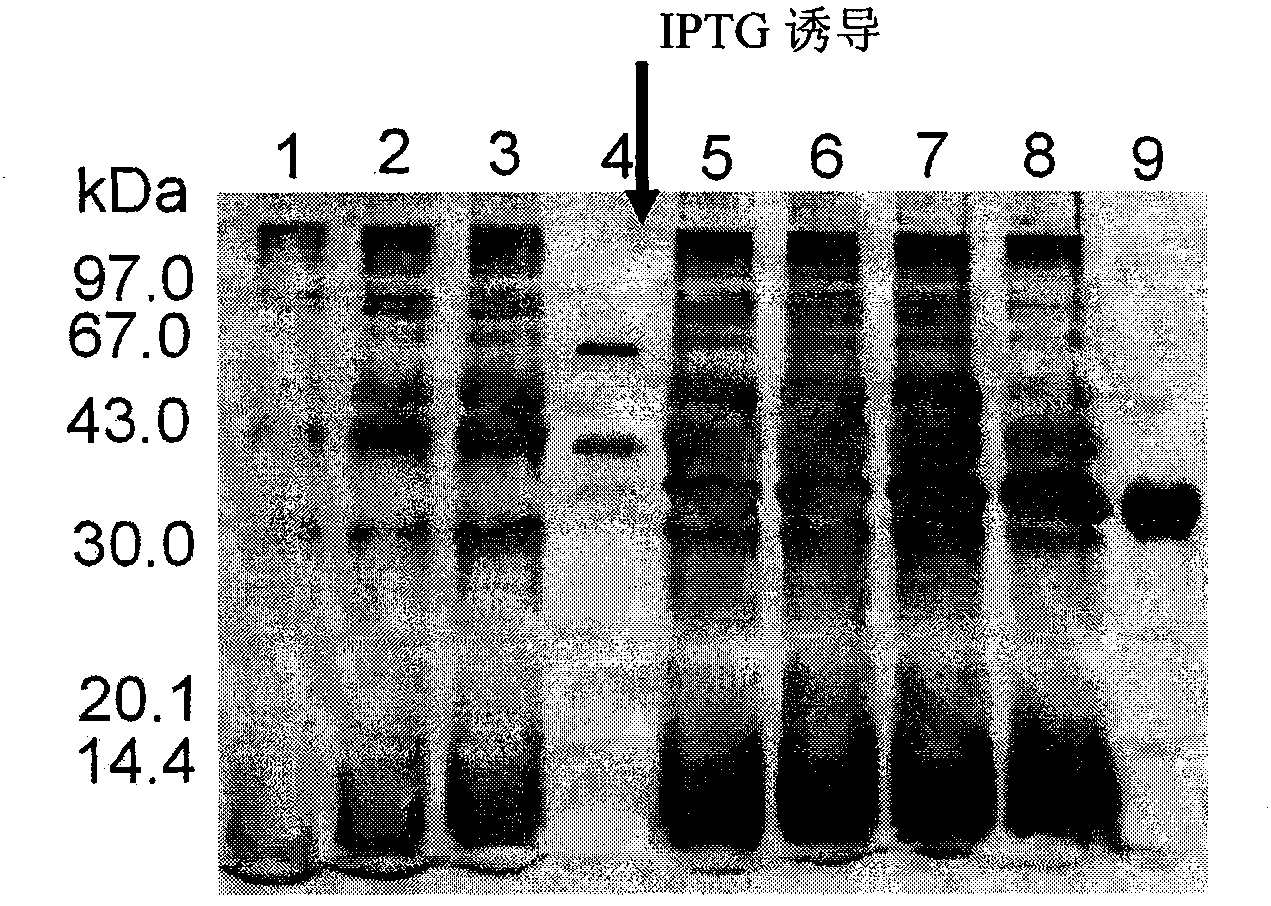
Examples
Embodiment 1A
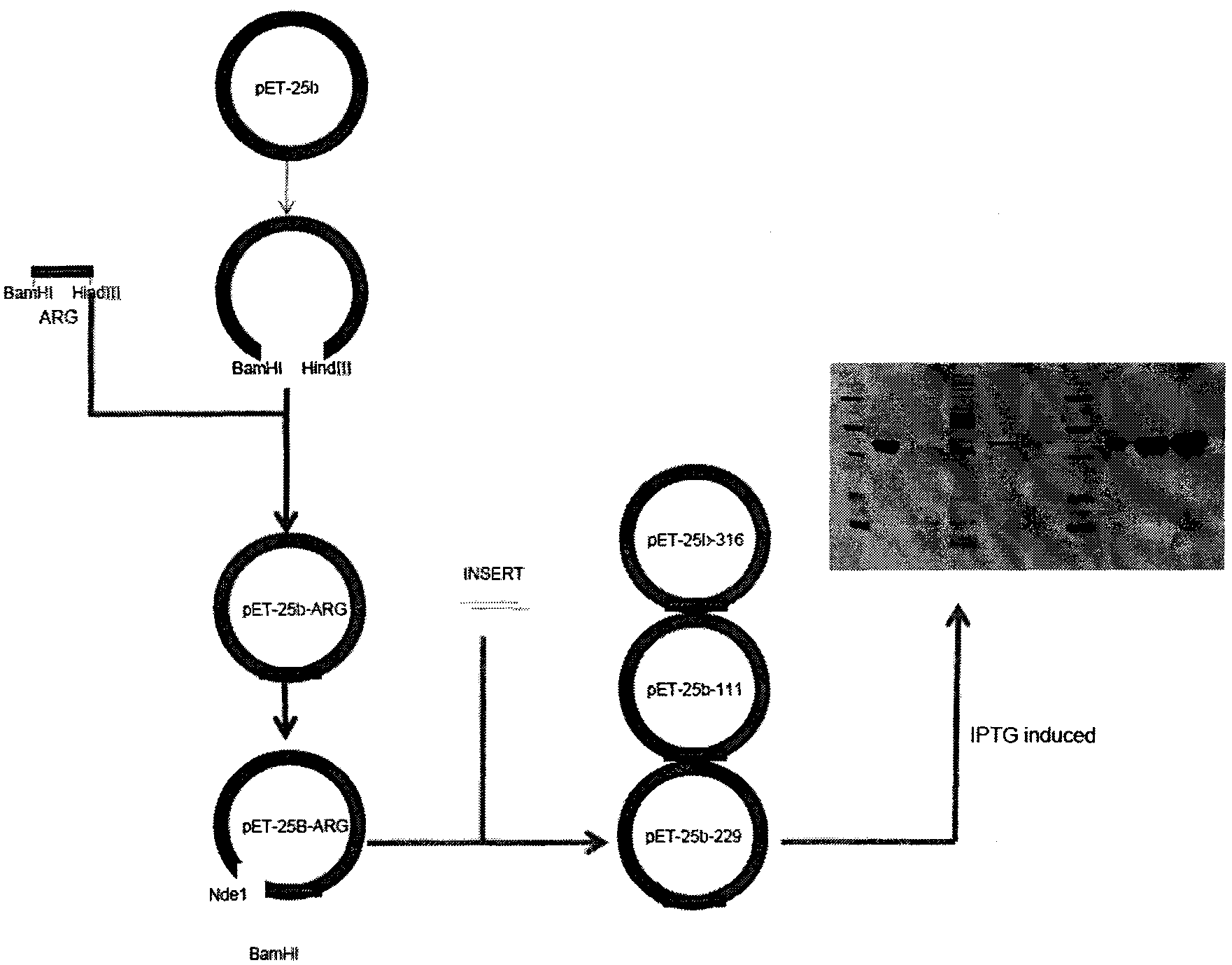
[0163] Embodiment 1A: Construction of NGR-arginase fusion protein recombinant strain
[0164](a) Isolation of the gene encoding human arginase Ⅰ
[0165] Primers were designed based on the published cDNA sequence of human arginase I (NM_000045). Polymerase chain reaction (PCR) was performed using Platinum I of TIANGEN to isolate the gene encoding human arginase I.
[0166] Primer ARG1: (5'-cg ggatcc cgccaagtccagaaccatag-3', SEQ ID NO: 4);
[0167] ARG2: (5'-ccc aagctt ttacttaggtgggttaaggtag-3', SEQ ID NO: 5) was purchased from SANGON. ARG1 contains a BamHI restriction enzyme recognition site (underlined), and primer ARG2 contains a HindIII restriction enzyme recognition site (underlined). These two primers (each at a final concentration of 400 nM) were added to 1 μl of a human liver cDNA library (Clontech) in a 0.2 ml cuvette. In addition, DNA Platinum Ⅰ (1 μl, 5 units), 4 kinds of deoxyribonucleic acids (each with a final concentration of 0.1 mM), reaction buffer (2.5...
Embodiment 1B
[0174] Example 1B: Construction of FCFWKTCT-arginase fusion protein recombinant strain
[0175] Design of primers containing the gene encoding FCFWKTCT
[0176] PRIMER2-SENSE (5' TATG TTCTGTTTCTGGAAGACGTGTACGG3', SEQ ID NO: 8);
[0177] PRIMER2-ANTISENSE (5' GATCC CGTACACGTCTTCCAGAAACAGAACA3', SEQ ID NO: 9) was purchased from SANGON, containing NdeI and BamHI sticky ends, these two primers (total 2μl, each with a final concentration of 50ng) were added to a 0.2ml small test tube, and PCR reaction buffer was added (2μl) and deionized water (16μl), a total of 20μl reaction system. Pairing was performed under the following conditions: 5 cycles (94°C, 1 min; 55°C, 1 min; 72°C, 1 min). The vector Pet-25b was treated with restriction enzymes BamHI and HindIII at 37°C for 2 hours. After completion, the reaction was subjected to agarose gel electrophoresis (1%), and a 6.5 kb DNA fragment was recovered from the gel using a common gel recovery kit (TIANGEN). The DNA product obta...
Embodiment 1C
[0178] Example 1C: Construction of DCLNGGTAVSNKYFSNIHWCN-arginase fusion protein recombinant strain
[0179] Design primers containing the gene encoding the urokinase fragment
[0180] PRIMER3-SENSE (5'GGAATTC CATATG GATTGTCTAAATGGAGGCACGGCAGTATCAAAACAAGTACTTCTCCAACAT3', SEQ ID NO: 10);
[0181] PRIMER3-ANTISENSE
[0182] (5′GAA GATCT GCTTCCAGAGCCACTCCCGTTACACCAGTGGATGTTGGAGAAGTACTTGTT3', SEQ ID NO: 11)
[0183] Purchased from SANGON, containing NdeI and BglII cohesive ends, these two primers (each with a final concentration of 50ng) were added to a 0.2ml small test tube, and DNA Platinum I (1 μl, 5 units), 4 kinds of deoxyribonucleic acid ( final concentration of 0.1 mM each) and reaction buffer (2.5 μl) and deionized water (17.5 μl). PCR was performed using the following conditions: pre-PCR (94°C, 5 minutes), 10 PCR cycles (94°C, 0.5 minutes; 58°C, 0.5 minutes; 72°C, 10 seconds), post-PCR (72°C, 7 minutes). The PCR product was treated with restriction enzymes BglII a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com