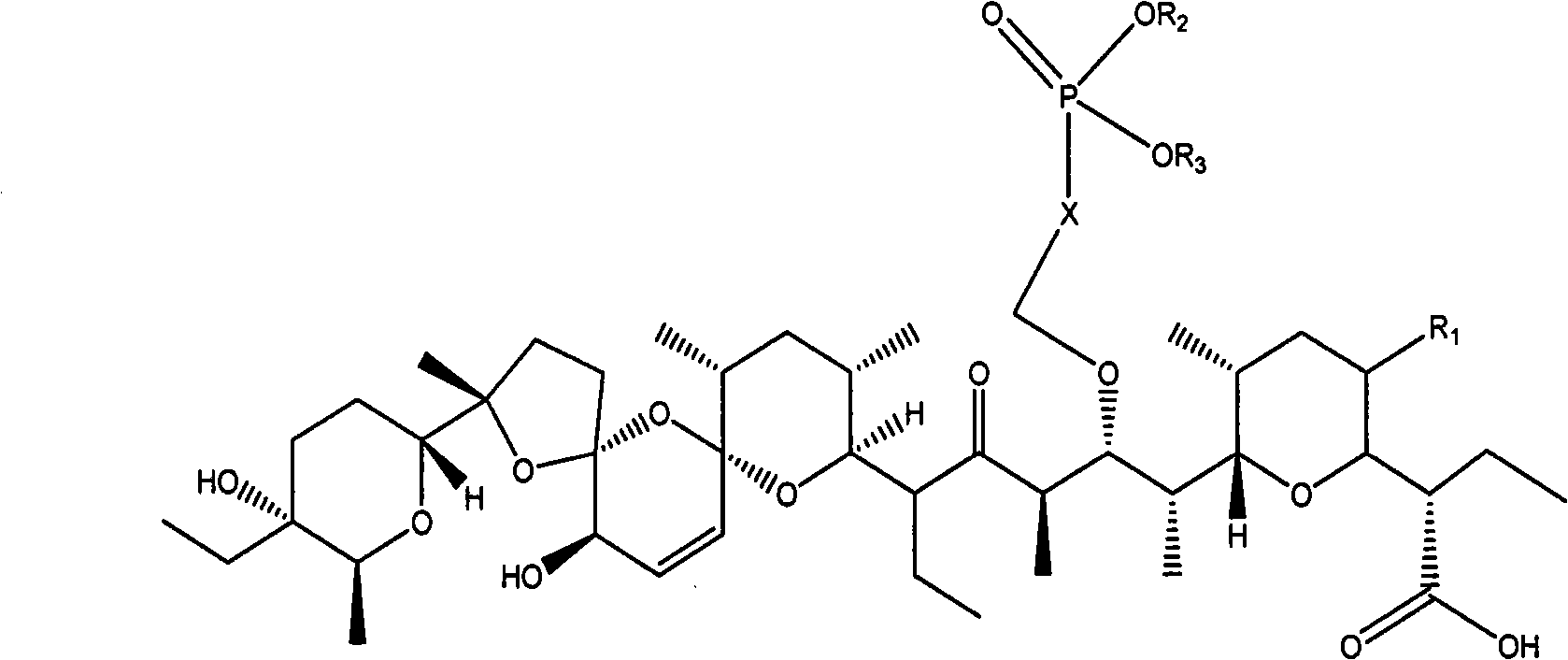
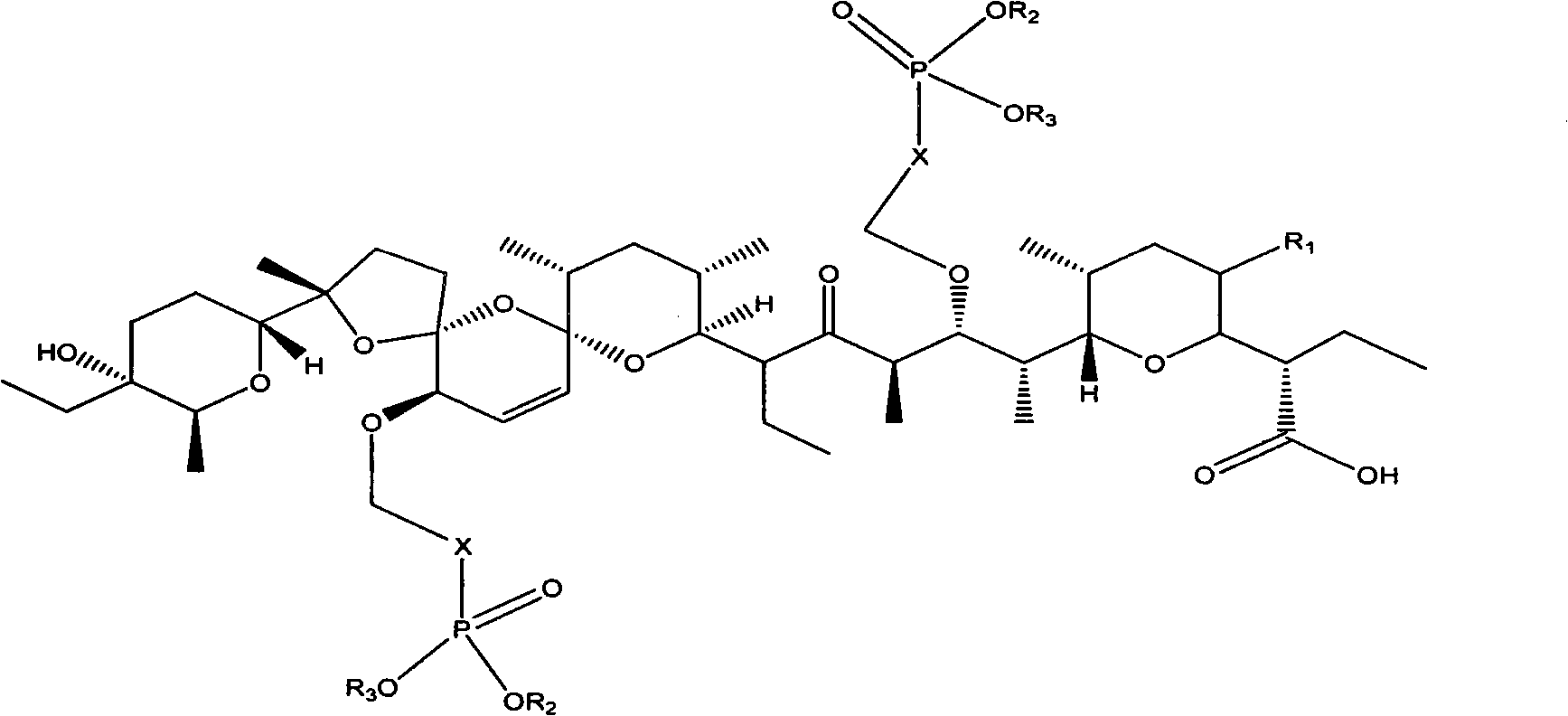
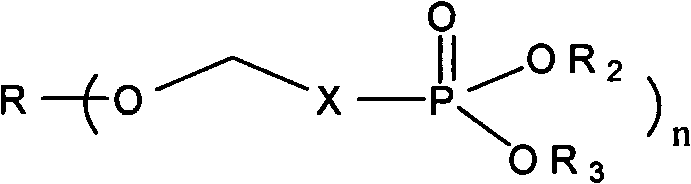Phosphoryl methyl salinomycin ether derivative and preparation method thereof
A technology of phosphoryl methyl salinomycin ether and methyl salinomycin ether, which is applied in the field of phosphoryl methyl salinomycin ether derivatives and its preparation, can solve the problem of poor water solubility of salinomycin and local pain and other problems, to achieve the effect of effectively killing cancer cells, preventing cancer metastasis, and high bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Synthesis of 9-Methylthiomethyl Salinomycin Ether
[0039]
[0040] Add salinomycin (10.74g, 14.3mmol) and dimethyl sulfoxide (250ml) into the reaction flask and stir to dissolve, then add acetic anhydride (125ml) and acetic acid (35ml) therein. The mixture was stirred vigorously at room temperature for 24 hours and followed by spot plate until the reaction was complete. Pour onto ice (800ml), stir for a further 30 minutes and extract with dichloromethane (4 x 100ml). The combined dichloromethane extracts were washed with water (2 x 100ml) and dried over magnesium sulfate. Dichloromethane was removed under reduced pressure and purified to give 9-methylthiomethyl salinomycin ether.
[0041] 1 H-NMR (D6-DMSO-D2O): δ0.96 (3CH3, t, 9H), 1.06 (4CH3, d, 12H), 1.16 (CH3, d, 3H), 1.21 (CH3, d, 3H), 1.31 (CH3, s, 3H), 1.44 (CH2, m, 2H), 1.56 (CH2, m, 2H), 1.57 (CH2, m, 2H), 1.64, 1.39 (CH2, m, 2H), 1.68, 1.43 ( CH2, m, 2H), 1.76 (CH, m, H), 1.84, 1.59 (CH2, m, 2H), 1.89,...
Embodiment 2
[0043] Synthesis of 9-phosphoryloxymethyl salinomycin ether dibenzyl ester
[0044]
[0045]Add N-iodosuccinimide (2.00 mL) to a well-stirred suspension of methylthiomethyl salinomycin ether (1.98 g, 2.44 mmol), powdered activated 4A molecular sieves (5 g) in tetrahydrofuran (20 mL) g, 95%, 8.44mmol) and a suspension of dibenzyl phosphate (2.20g, 7.83mmol) in dichloromethane (12ml). The mixture was stirred vigorously at room temperature for 30 minutes and followed by spot plate until the reaction was complete. Filter and dilute with ethyl acetate (300ml). The solution was washed with aqueous sodium thiosulfate (10%, 2 x 15ml), water (2 x 20ml), brine (50ml) and dried over magnesium sulfate. The mixture was filtered, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel flash column chromatography to obtain 9-phosphoryloxymethyl salinomycin ether dibenzyl ester.
[0046] 1 H-NMR (D6-DMSO-D2O): δ0.96 (3CH3, t, 9H), 1.06 (4CH3, d...
Embodiment 3
[0048] Synthesis of 9-phosphoryloxymethyl salinomycin ether
[0049]
[0050] To a solution of 9-phosphoryloxymethyl salinomycin ether dibenzyl ester (0.81 g, 0.78 mmol) in tetrahydrofuran (100 mL) and water (5 mL) was added palladium on carbon (10%, 500 mg). The mixture was stirred under hydrogen (1 atm) for 35 minutes and followed by spot plate until the reaction was complete. The catalyst was removed by filtration through celite. Celite was then washed with tetrahydrofuran (300ml) and the combined filtrates were evaporated under reduced pressure. The solid formed was washed with diethyl ether (2 x 20ml), hexane (50ml), dried in vacuo and dissolved in hot methanol (60ml). The solution was filtered and concentrated under reduced pressure to a volume of about 10 ml. After standing at room temperature for 1 hour, the solution was placed in the refrigerator overnight. The crystalline precipitate formed overnight was filtered off and dried in vacuo to yield the product. T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com