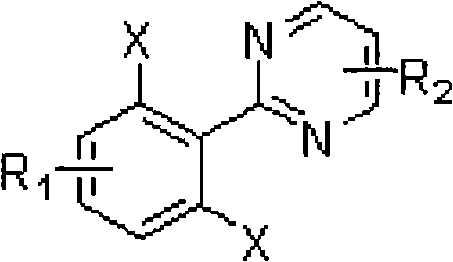
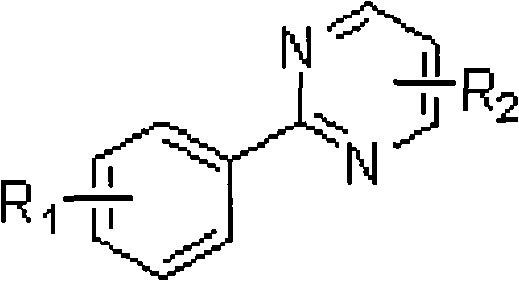
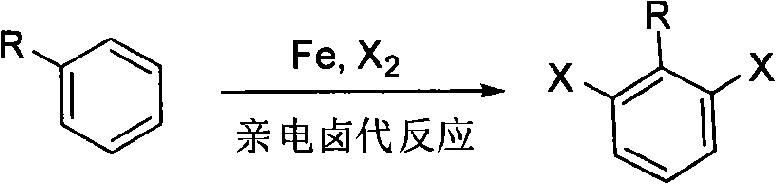Ortho-position dihalogen substitution compound of aryl pyrimidine and preparing method thereof
A technology for arylpyrimidines and compounds, which is applied to the ortho-dihalogenated compounds of arylpyrimidines and their preparation fields, can solve the problems of harshness, large limitations, and low reaction yields, and achieves mild conditions, environment-friendly reaction, and operation. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: Preparation of 2-(2,6-dichlorophenyl)pyrimidine
[0041] 2-(2,6-dichlorophenyl)pyrimidine adopts the following steps: 1. add 10 grams of 2-phenylpyrimidine, 250 milligrams of palladium acetate, 22 grams of additive copper trifluoroacetate, and 35 grams of Calcium chloride, 150ml acetic acid, heated to 80°C. Use thin layer chromatography to track the reaction until the reaction raw material 2-phenylpyrimidine disappears; ② After the reaction, remove the solvent with a rotary evaporator, add saturated sodium bicarbonate solution to the system, extract the product with ethyl acetate, and dry Remove the solvent with a rotary evaporator to obtain a crude product; ③ the crude product is purified by column chromatography (petroleum ether: ethyl acetate=20: 1) to obtain 14.4 grams of 2-(2,6-dichlorophenyl) pyrimidine, producing The rate is 96%. Melting point: 130-132°C.
[0042] IR (KBr, cm -1 ): 3044, 1587, 1562, 1466, 1430, 1406, 780.
[0043] 1 H NMR (CDCl ...
Embodiment 2
[0045] Example 2: Preparation of 2-(2,6-dichloro-4-methylphenyl)pyrimidine
[0046] 2-(2,6-dichloro-4-methylphenyl) pyrimidine adopts the following steps: 1. add 10 grams of 2-(4-methylphenyl) pyrimidine, 370 mg of palladium acetate in a 250 ml round bottom flask , 17 grams of additive copper trifluoroacetate, 30 grams of calcium chloride, 150 milliliters of acetic acid, heated to 80 ° C. Track the reaction with thin-layer chromatography until the reaction raw material 2-(4-methylphenyl)pyrimidine disappears; 2. After the reaction is finished, remove the solvent with a rotary evaporator, add saturated sodium bicarbonate solution to the system, and use ethyl acetate Ester extraction product, after drying, remove the solvent with a rotary evaporator to obtain a crude product; ③The crude product is purified by column chromatography (petroleum ether:ethyl acetate=20:1) to obtain 13.6 grams of 2-(2,6-dichloro -4-methylphenyl)pyrimidine, the yield was 97%. Melting point: 117-119°C...
Embodiment 3
[0050] Example Three: Preparation of 2-(2,6-dibromo-4-methylphenyl)pyrimidine
[0051] 2-(2,6-dibromo-4-methylphenyl) pyrimidine adopts the following steps: 1. add 10 grams of 2-(4-methylphenyl) pyrimidine, 500 mg of palladium acetate in a 250 ml round bottom flask , 50 grams of additive copper trifluoroacetate, 70 grams of calcium bromide, 150 milliliters of acetic acid, heated to 80 ° C, followed the reaction with thin-layer chromatography until the reaction raw material 2-(4-methylphenyl) pyrimidine disappeared; ② After the reaction was finished, remove the solvent with a rotary evaporator, add saturated sodium bicarbonate solution in the system, extract the product with ethyl acetate, remove the solvent with a rotary evaporator after drying, and obtain the crude product; ③ the crude product was analyzed by column chromatography (petroleum ether:ethyl acetate=20:1) to obtain 18.3 g of 2-(2,6-dibromo-4-methylphenyl)pyrimidine with a yield of 95%. Melting point: 142-144°C. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com