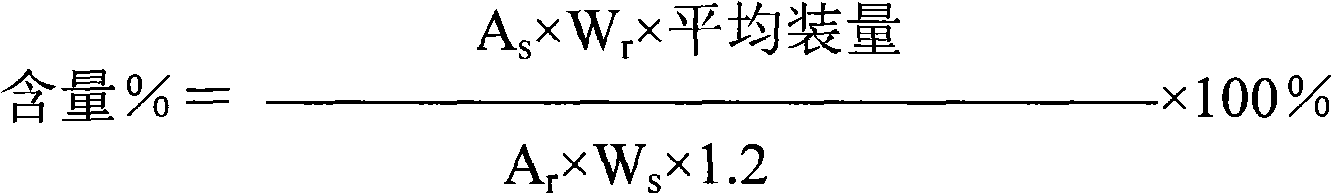Prefreezing method in preparing injection-used reduced glutathione with freeze drying method
A technology of glutathione and reduced form, which is applied in freeze-dried transportation, tripeptide components, anti-toxins, etc., can solve problems such as difficult manipulation, unstable quality, and high scrap rate, and achieve the goal of overcoming manipulation difficulties, ensuring medication safety, The effect of improving product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
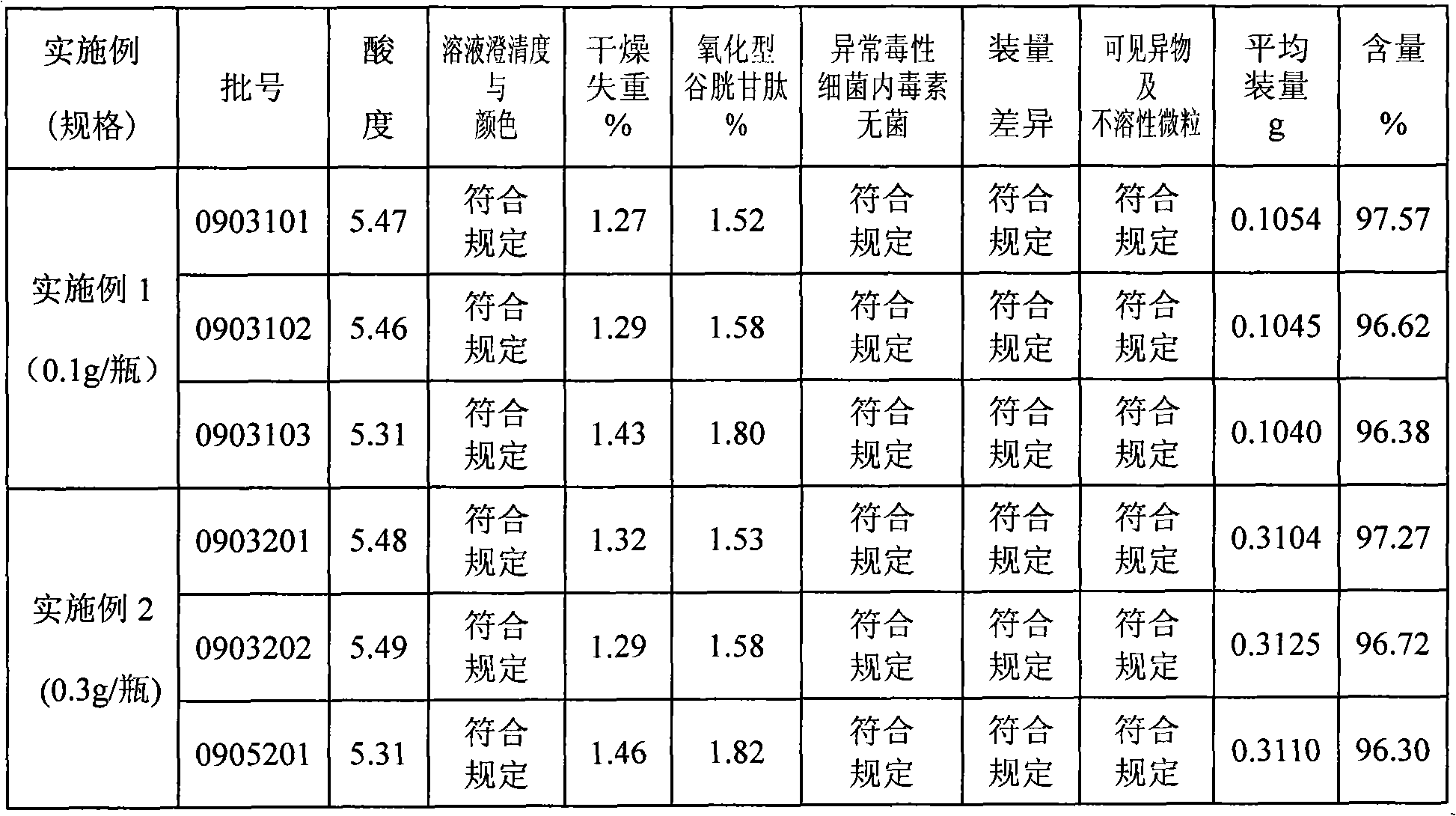
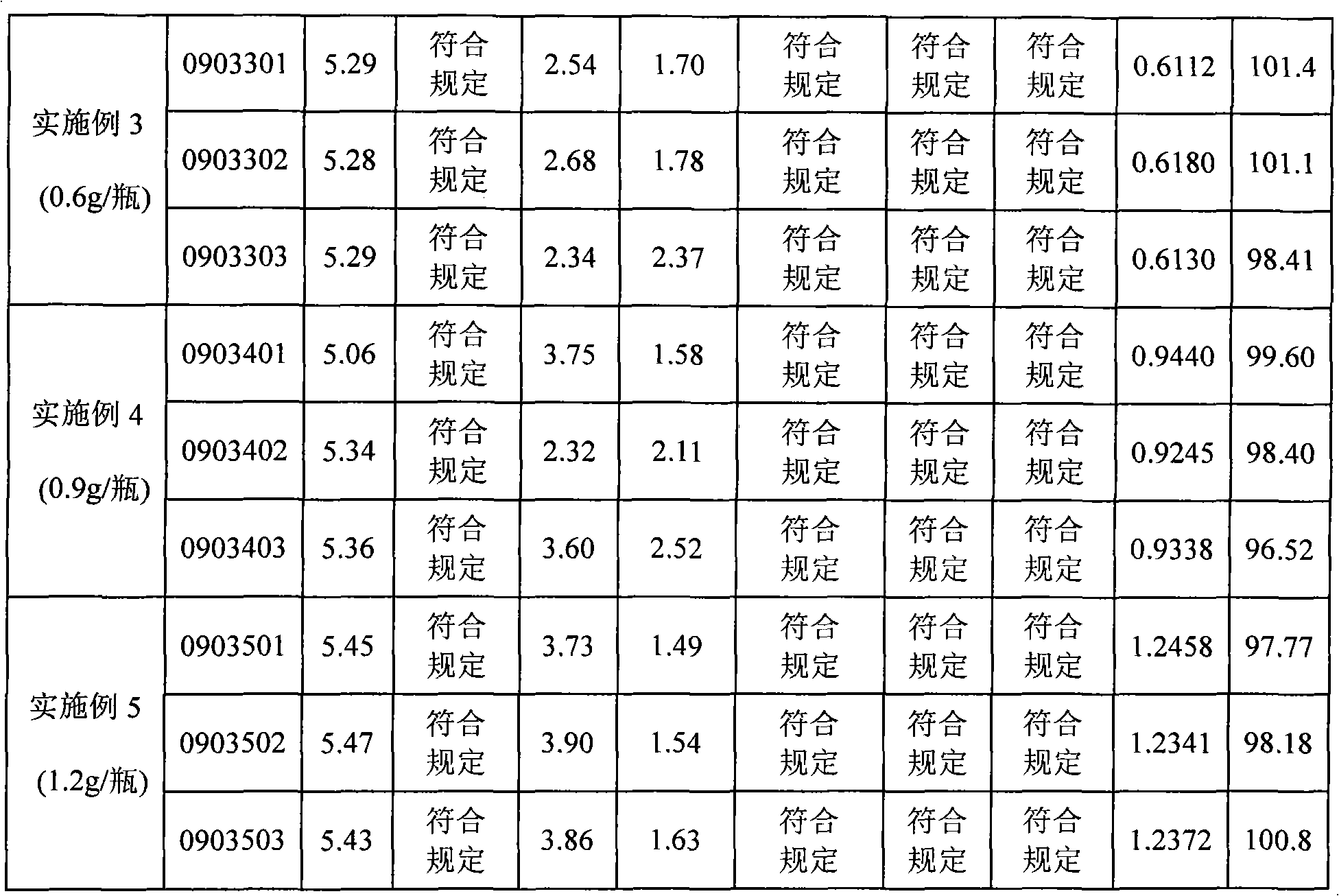
[0038] First add about 25L of water for injection into the sterilized liquid preparation pot, control the water temperature below 40°C, add 4.1Kg of reduced glutathione, stir evenly, add about 5.4L of 10% sodium hydroxide, and stir at the same time to dissolve and clarify , add water for injection to a total of 82L, and adjust the pH value to 4.8-5.8. After the analysis is qualified, the liquid medicine is first filtered with a 0.45 μm filter, and then sterilized with a 0.22 μm microporous filter membrane. Pack in 10ml control bottles, 0.1g / 2ml per bottle, check the accuracy of the filling every half hour. After the filling is finished, the machine will automatically half-stopper, then put the filled product into the suction chassis and send it into the freeze-drying box in time, and close the box door. Reduce the product temperature from room temperature to -40°C within 1.5 to 2 hours, and keep it warm for 1 hour; within 2 hours, raise the product temperature from -40°C to -1...
Embodiment 2
[0040] First add about 30L of water for injection into the sterilized liquid preparation pot, control the water temperature below 40°C, add 12.3Kg of reduced glutathione, stir evenly, add about 16.2L of 10% sodium hydroxide, and stir at the same time to dissolve and clarify , add water for injection to a total of 82L, and adjust the pH value to 4.8-5.8. After the analysis is qualified, the liquid medicine is first filtered with a 0.45 μm filter, and then sterilized with a 0.22 μm microporous filter membrane. Pack in 10ml control bottles, 0.3g / 2ml per bottle, check the accuracy of the filling every half hour. After the filling is finished, the machine will automatically half-stopper, then put the filled product into the suction chassis and send it into the freeze-drying box in time, and close the box door. Reduce the product temperature from room temperature to -40°C within 1.5 to 2 hours, and keep it warm for 1 hour; within 2 hours, raise the product temperature from -40°C to ...
Embodiment 3
[0042] First add about 30L of water for injection into the sterilized liquid preparation pot, control the water temperature below 40°C, add 24.6Kg of reduced glutathione, stir evenly, add about 32.4L of 10% sodium hydroxide, and stir at the same time to dissolve and clarify , add water for injection to a total of 82L, and adjust the pH value to 4.8-5.8. After the analysis is qualified, the liquid medicine is first filtered with a 0.45 μm filter, and then sterilized with a 0.22 μm microporous filter membrane. Packed in 10ml control bottles, 0.6g / 2ml per bottle, check the accuracy of the filling every half hour. After the filling is finished, the machine will automatically half-stopper, then put the filled product into the suction chassis and send it into the freeze-drying box in time, and close the box door. Reduce the product temperature from room temperature to -40°C within 1.5 to 2 hours, and keep it warm for 1 hour; within 2 hours, raise the product temperature from -40°C t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com