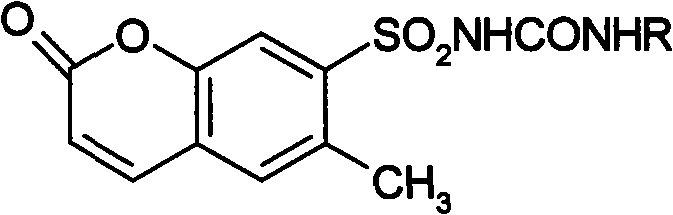
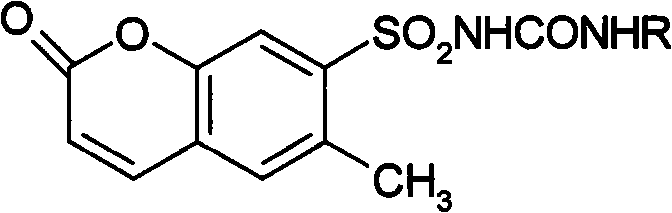
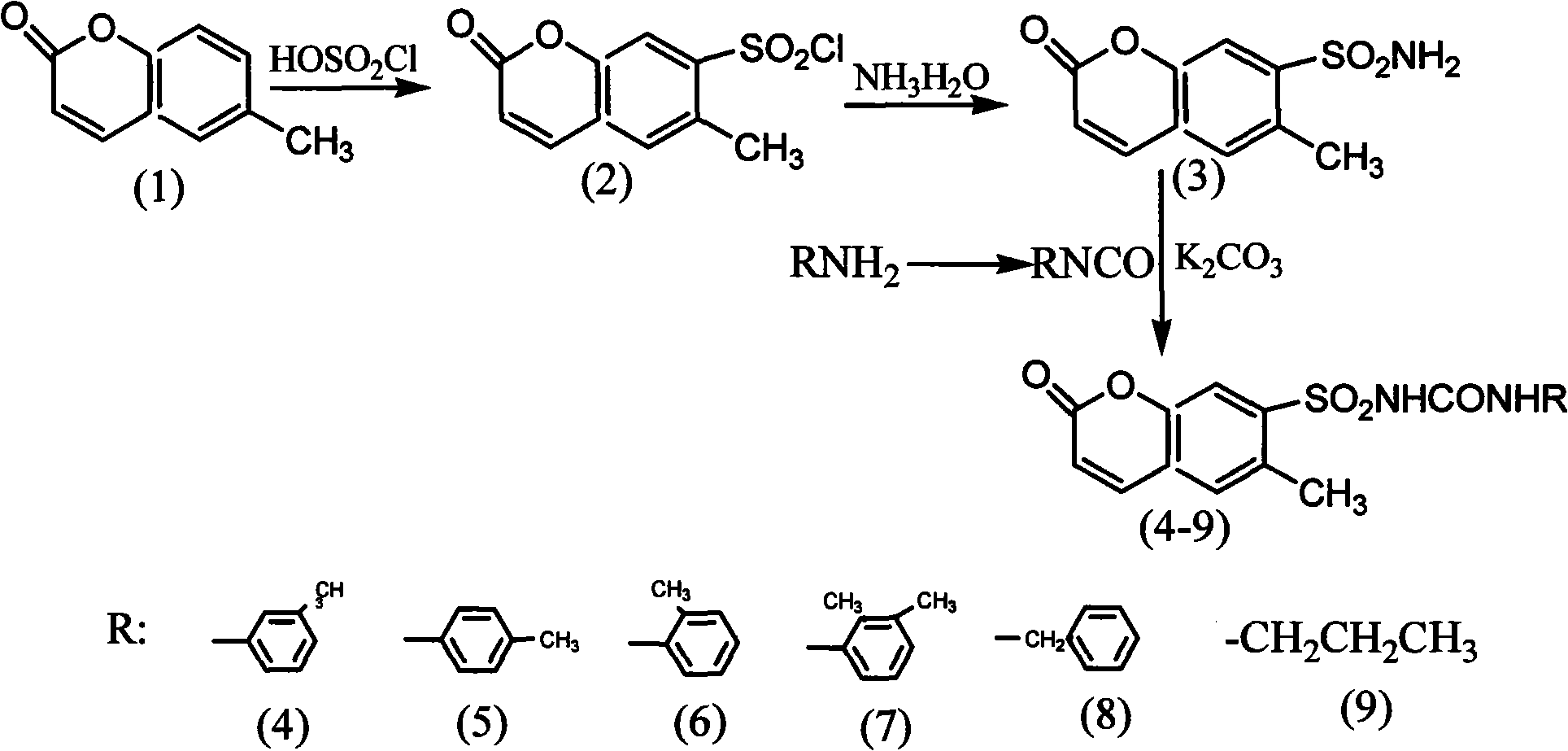
6- methylcoumarin-7-sulfonylurea compound and synthetic method thereof
A technology of methyl coumarin and sulfonylurea, which is applied in the directions of organic chemistry, drug combination, metabolic diseases, etc., to achieve the effect of simple and easy synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: 6-methylcoumarinsulfonyl chloride (2)
[0029] Add 10 g of 6-methylcoumarin (1) (0.06 mol) to 9 mL of chlorosulfonic acid (0.15 mol) in an ice-water bath, keep the temperature below 10 ° C, after the addition is complete, stir at room temperature until the solid is completely dissolved, and then Heat to 90-95°C to continue the reaction for 4h, cool to room temperature, pour into 20g of crushed ice under vigorous stirring (800r / min), filter with suction to obtain a solid, wash twice with water to obtain crude 6-methylcoumarinsulfonyl chloride ( 2) 16g is directly used in the next step reaction.
Embodiment 2
[0030] Embodiment 2: 6-methylcoumarin sulfonamide (3)
[0031] Add 16 g of the obtained 6-methylcoumarin sulfonyl chloride crude product (2) to 15 mL of 28% concentrated ammonia water cooled by ice water, stir at room temperature for 8 h, adjust the pH to neutral with dilute hydrochloric acid, and recrystallize from water to obtain 2.30 g of white powder that is It is 6-methylcoumarin sulfonamide (3), the yield is 16%, mp.245~247℃. ESI-MS: 262[M+Na] +
Embodiment 3
[0032] Embodiment 3: the synthetic general method of isocyanate
[0033] Dissolve 12g of triphosgene (0.40mol) in 50mL of chloroform, stir and dissolve, then add dropwise 10mL of the chloroform solution of the corresponding amine compound (0.02moL) under ice bath cooling, the amine compound described here is 2-methylaniline , 3-methylaniline, 4-methylaniline, 2,3-dimethylaniline, benzylamine or n-propylamine, after addition, react at room temperature for 1h, then heat to reflux for 6h, and distill to obtain the corresponding isocyanate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com