Cefetamet pivoxil hydrochloride capsule and preparation method thereof
A technology of ceftamet pivoxil hydrochloride and capsules, which is applied in the field of ceftamet pivoxil hydrochloride capsules and its preparation, can solve problems such as relatively large impact on product quality, easy degradation of active ingredients, and conflicting psychology, and achieve good dissolution effect and dissolution Excellent performance, the effect of improving viscosity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
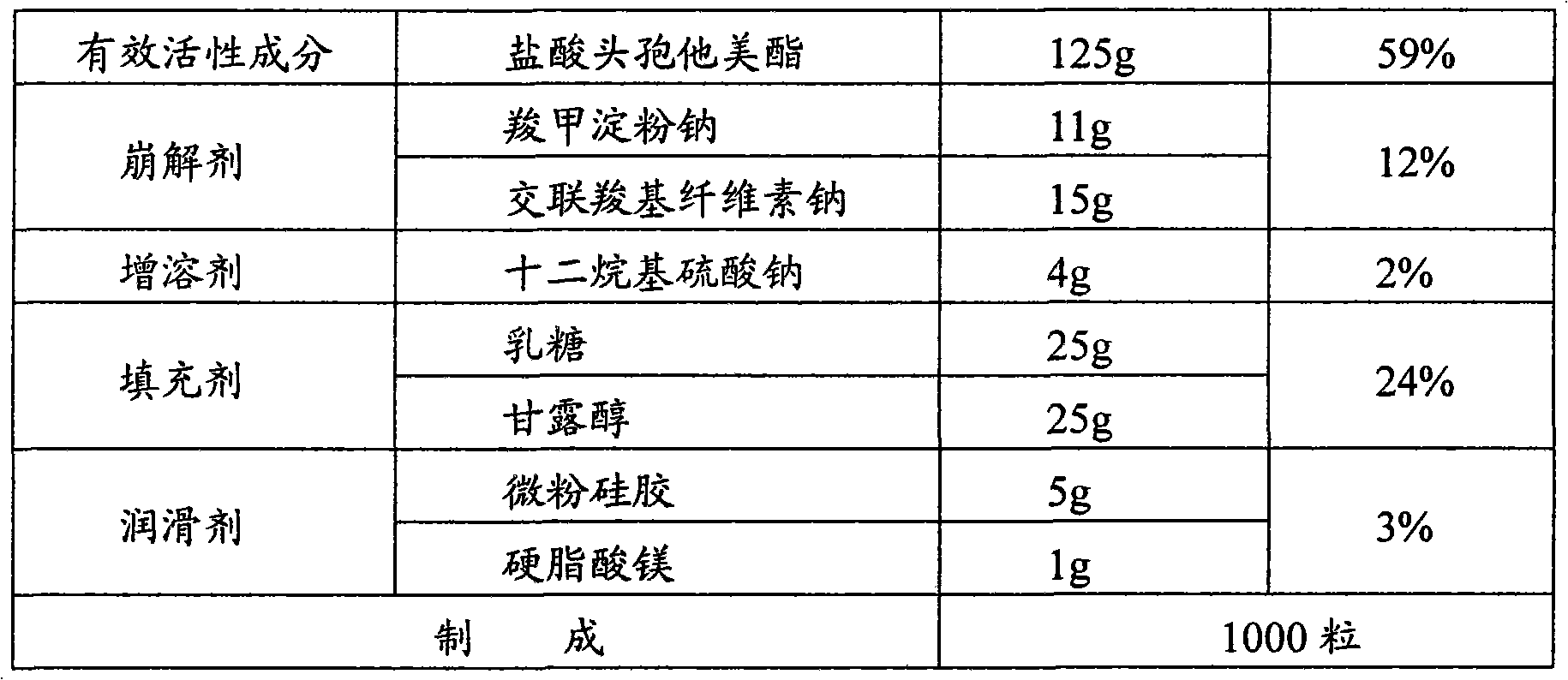
[0039]
[0040] Dry sodium carboxymethyl starch at 120°C for 4 hours, seal it with a double-layer plastic bag and put it in a stainless steel turnover bucket for later use; dry the lactose at 80°C for 4 hours, seal it with a double-layer plastic bag and put it in a stainless steel turnover barrel for later use; After the alcohol is dried at 80°C for 4 hours and passed through an 80-mesh sieve, it is sealed with a double-layer plastic bag and put into a stainless steel turnover bucket for later use.
[0041] Put the above-mentioned dried sodium carboxymethyl starch, lactose and mannitol, croscarmellose sodium, sodium lauryl sulfate and micropowder silica gel into the mixer and pre-mix for 10 minutes. After mixing evenly, add 1 / 2 The prescription amount of ceftazidime pivoxil hydrochloride was premixed for 10 minutes, and then 1 / 2 of the prescription amount of ceftazime pivoxil hydrochloride was added and mixed evenly to obtain a mixed material.
[0042]The above mixed materi...
Embodiment 2
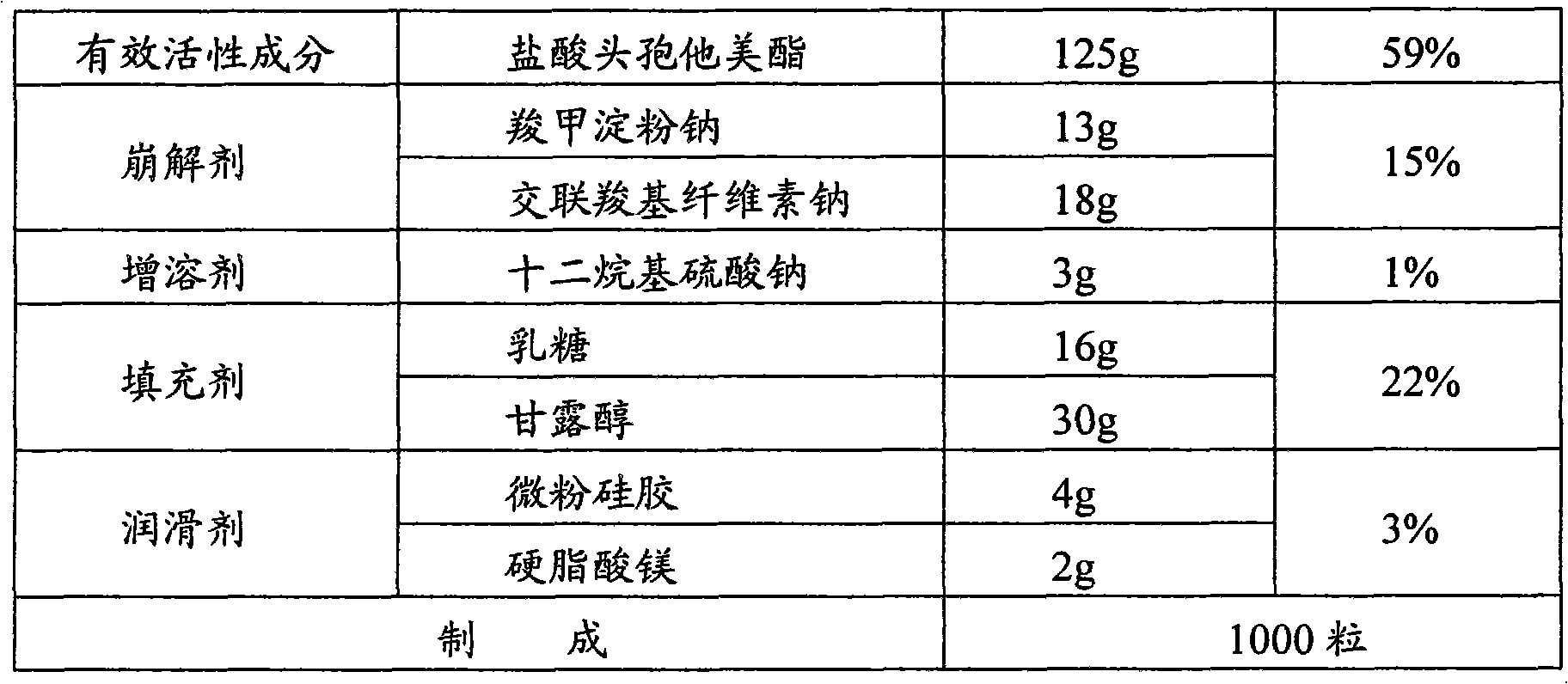
[0045]
[0046] The preparation method is the same as in Example 1.
Embodiment 3
[0048]
[0049] The preparation method is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com