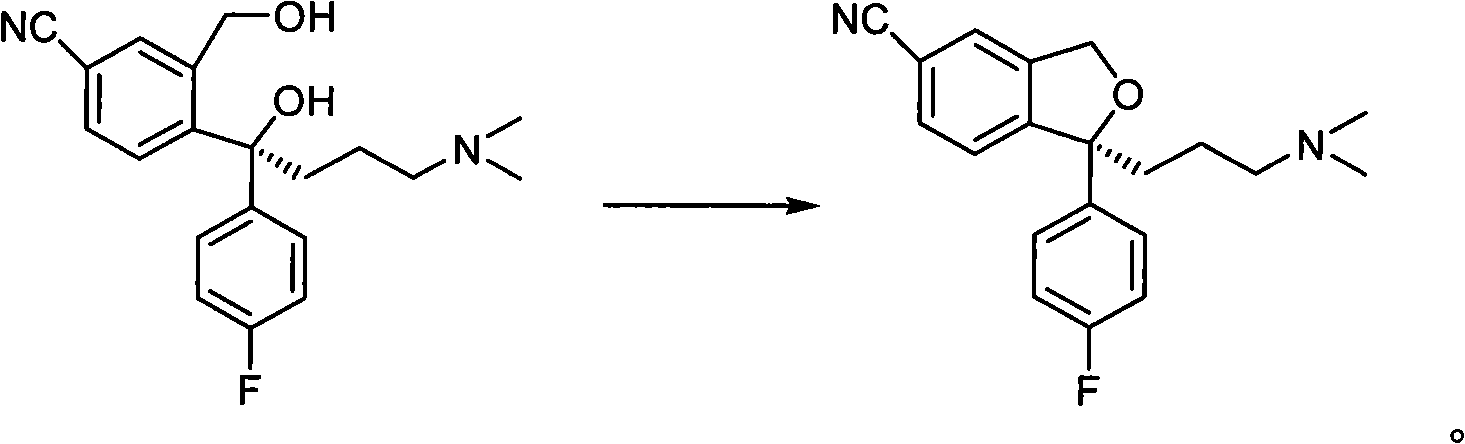
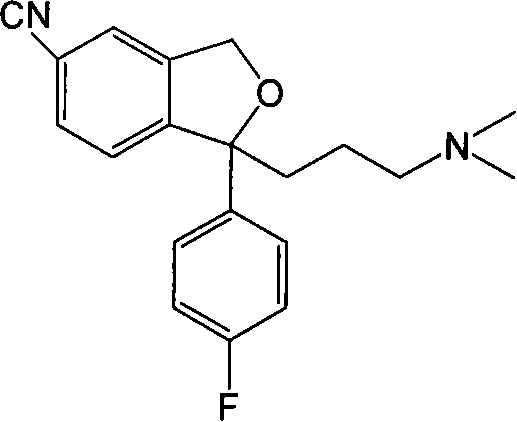
Method for preparing escitalopram
A coordination and phosphine compound technology, which is applied in nervous system diseases, organic chemistry, drug combination, etc., can solve problems such as low yield, racemization or isomerization, and achieve high yield, mild conditions, and optical purity and the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Preparation of Escitalopram
[0031] In a reaction flask under nitrogen protection, add S-4-[4-(dimethylamino)-1-(4'-fluorophenyl)-1-hydroxybutyl]-3-(hydroxymethyl)-cyanide 34.2g (0.1mol) of benzene (S-diol ee value 98.5%) and 340ml of anhydrous tetrahydrofuran were added at room temperature with 57.6g (0.22mol) of triphenylphosphine and 10.2g (0.22mol) of 98% formic acid, Add 44.5 g (0.22 mol) of diisopropyl azodicarboxylate dropwise, react at room temperature of 20°C, wait for TLC to detect the end of the reaction (2 hours), evaporate the tetrahydrofuran to dryness under reduced pressure, and add toluene to the remaining oil. 200ml, cooled to 0 degrees and stirred, a large amount of white solid was precipitated, suction filtration, 200ml of 1N dilute hydrochloric acid was added to the filtrate, stirred, and the liquid was separated. Extract with ethyl acetate 100ml*3, combine the organic layers, wash with saturated brine, dry over anhydrous sodium sulfate, ...
Embodiment 2
[0034] Example 2 Preparation of Escitalopram
[0035] In a reaction flask under nitrogen protection, add S-4-[4-(dimethylamino)-1-(4'-fluorophenyl)-1-hydroxybutyl]-3-(hydroxymethyl)-cyanide 34.2g (0.1mol) of benzene (S-diol ee value 98.5%) and 340ml of anhydrous tetrahydrofuran were added at room temperature with 16.7g (0.22mol) of trimethylphosphine and 10.2g (0.22mol) of 98% formic acid, Add 44.5 g (0.22 mol) of diisopropyl azodicarboxylate dropwise, react at 30°C, wait for TLC to detect the end of the reaction (2 hours), evaporate the tetrahydrofuran to dryness under reduced pressure, and add 200 ml of toluene to the remaining oil. , cooled to 0 degrees and stirred, a large amount of white solid was precipitated, suction filtration, 200ml of 1N dilute hydrochloric acid was added to the filtrate, stirred, and the liquid was separated. Extract with 100ml*3 of ethyl ester, combine the organic layers, wash with saturated brine, dry over anhydrous sodium sulfate, filter with su...
Embodiment 3
[0037] Example 3 Preparation of Escitalopram
[0038]In a reaction flask under nitrogen protection, add S-4-[4-(dimethylamino)-1-(4'-fluorophenyl)-1-hydroxybutyl]-3-(hydroxymethyl)-cyanide 34.2g (0.1mol) of benzene (S-diol ee value 98.5%) and 340ml of anhydrous tetrahydrofuran were added at room temperature with 15.2g (0.2mol) of trimethylphosphine and 9.2g (0.2mol) of 98% formic acid, Add 19.2 g (0.2 mol) of diethyl azodicarboxylate dropwise, react at room temperature of 25°C, and wait for TLC to detect the end of the reaction (2 hours), evaporate the tetrahydrofuran to dryness under reduced pressure, and add 200 ml of toluene to the remaining oil. , cooled to 0 degrees and stirred, a large amount of white solid was precipitated, suction filtration, 200ml of 1N dilute hydrochloric acid was added to the filtrate, stirred, and the liquid was separated. Extract with 100ml*3 of ethyl ester, combine the organic layers, wash with saturated brine, dry over anhydrous sodium sulfate,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com