Cytidine disodium triphosphate freeze-dried powder-injection for injection
A technology of cytidine triphosphate disodium and freeze-dried powder injection, which is applied in the field of prescription of cytidine triphosphate disodium freeze-dried powder injection, which can solve the problems of content reduction and unstable content of cytidine triphosphate disodium , to achieve the effects of easy operation, broad application prospects and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Preparation prescription:
[0022]
[0023] Preparation Process:
[0024] (1) Weigh the prescribed amount of dextran 40, put it in a liquid tank, add water for injection to make a 10% solution, heat to boil, stir to dissolve. Add water for injection to 80% of the total volume and cool the solution to below 40°C for use.
[0025] (2) Pour the accurately weighed cytidine disodium triphosphate, guanidine carbonate and arginine into the above solution, stir to dissolve them, and then adjust the pH value with dilute hydrochloric acid to control the pH value between 6.0 and 6.5 . After the pH value is adjusted to the control range, add the weighed activated carbon (0.25%, g / L) into the liquid medicine, stir well, and let it stand for 15 minutes. Filter and decarbonize the liquid medicine and press it into the dilute tank, and add injection Water to the full amount, take samples for intermediate inspection, after the pH value of the liquid medicine is checked and passed,...
Embodiment 2
[0031] Preparation prescription:
[0032]
[0033] Prepared by the technique of Example 1.
Embodiment 3
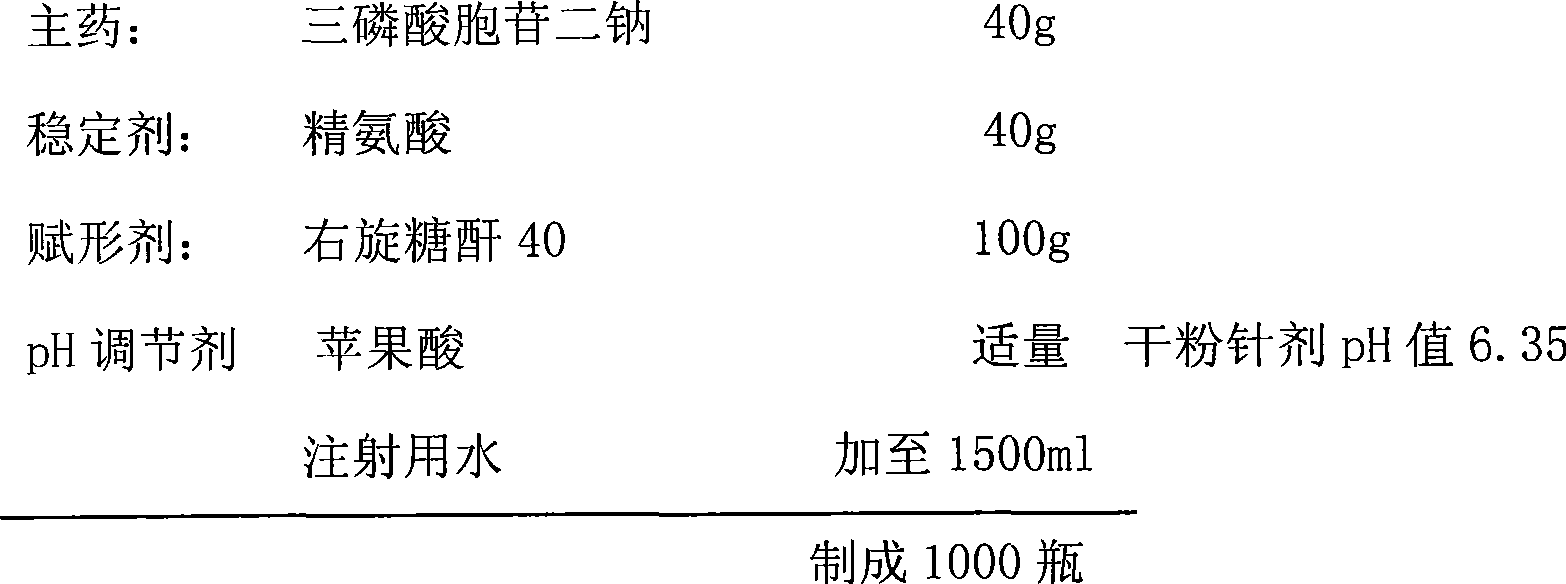
[0035] Preparation prescription:
[0036]
[0037] Prepared by the technique of Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com