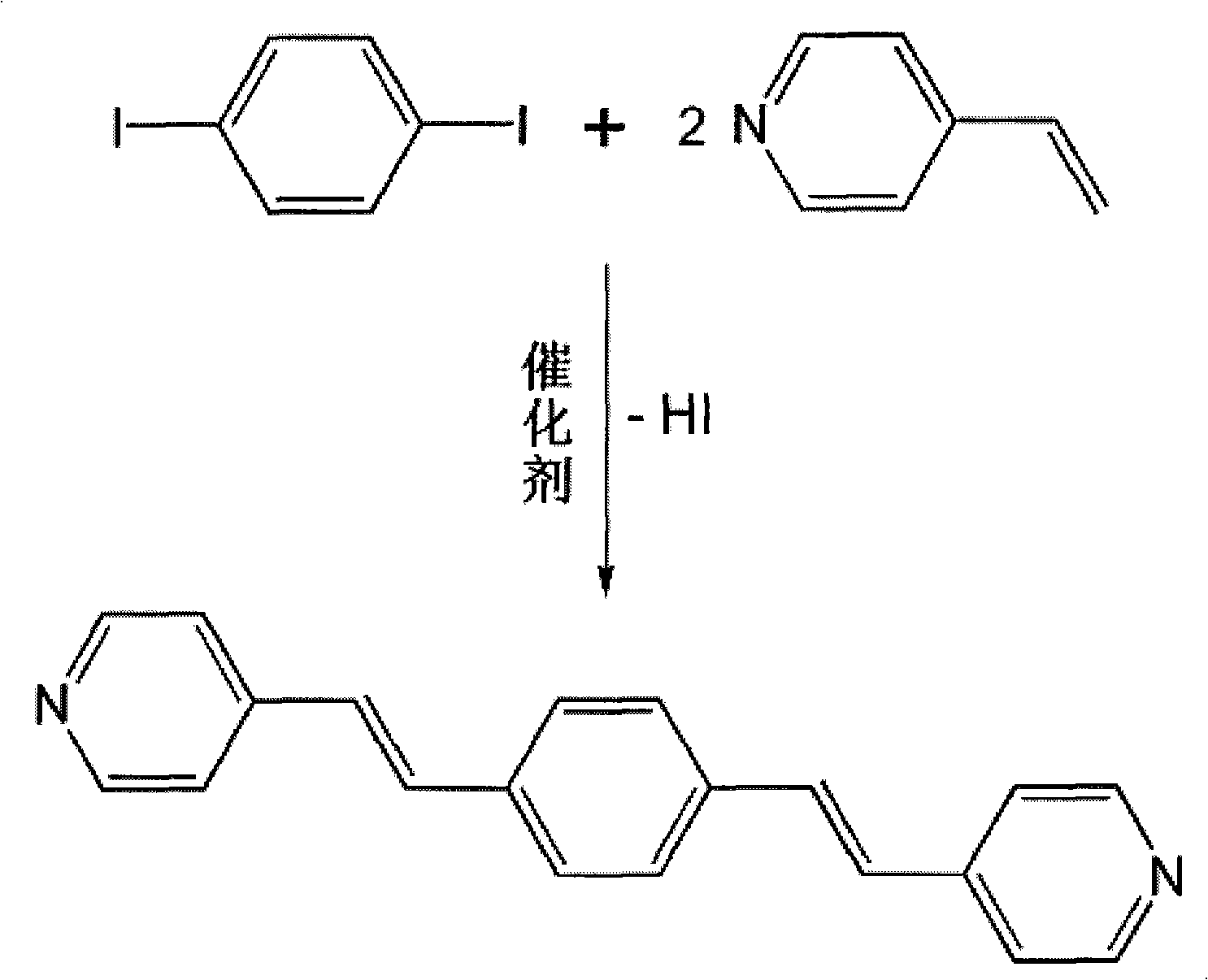
Method for preparing beta, beta'-binary (4-pyridyl) divinylbenzene
A divinylbenzene and pyridyl technology, applied in the beta field, can solve the problems of harsh reaction conditions, many reaction steps, and long reaction time, and achieve the effects of short reaction time, simple device and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] First, the palladium catalyst was prepared according to techniques known to those skilled in the art. Palladium chloride and excess hydrazine hydrate were mixed and stirred for about 2 hours, filtered, and the obtained gray solid was vacuum-dried and sealed for storage.
[0027] 3.30 g (10 mmol) of p-diiodobenzene was dissolved in 15 mL of N, N'-dimethylformamide, and 30 mg of palladium catalyst, 2.61 g (25 mmol) of 4-vinylpyridine and 2.53 g of triethylamine were added thereto. g (25mmol), stirring at a temperature of about 100°C, during the reaction process, point the plate to estimate the reaction conversion rate, and the reaction is complete in 6 hours. For the reaction process, see figure 1 .
[0028] After the reaction was terminated, the above-mentioned reaction bottle was vacuumed to remove the reaction solvent N,N'-dimethylformamide to obtain a brownish-yellow solid. The tan solid was washed with water and dried, and then subjected to silica gel column chromat...
Embodiment 2
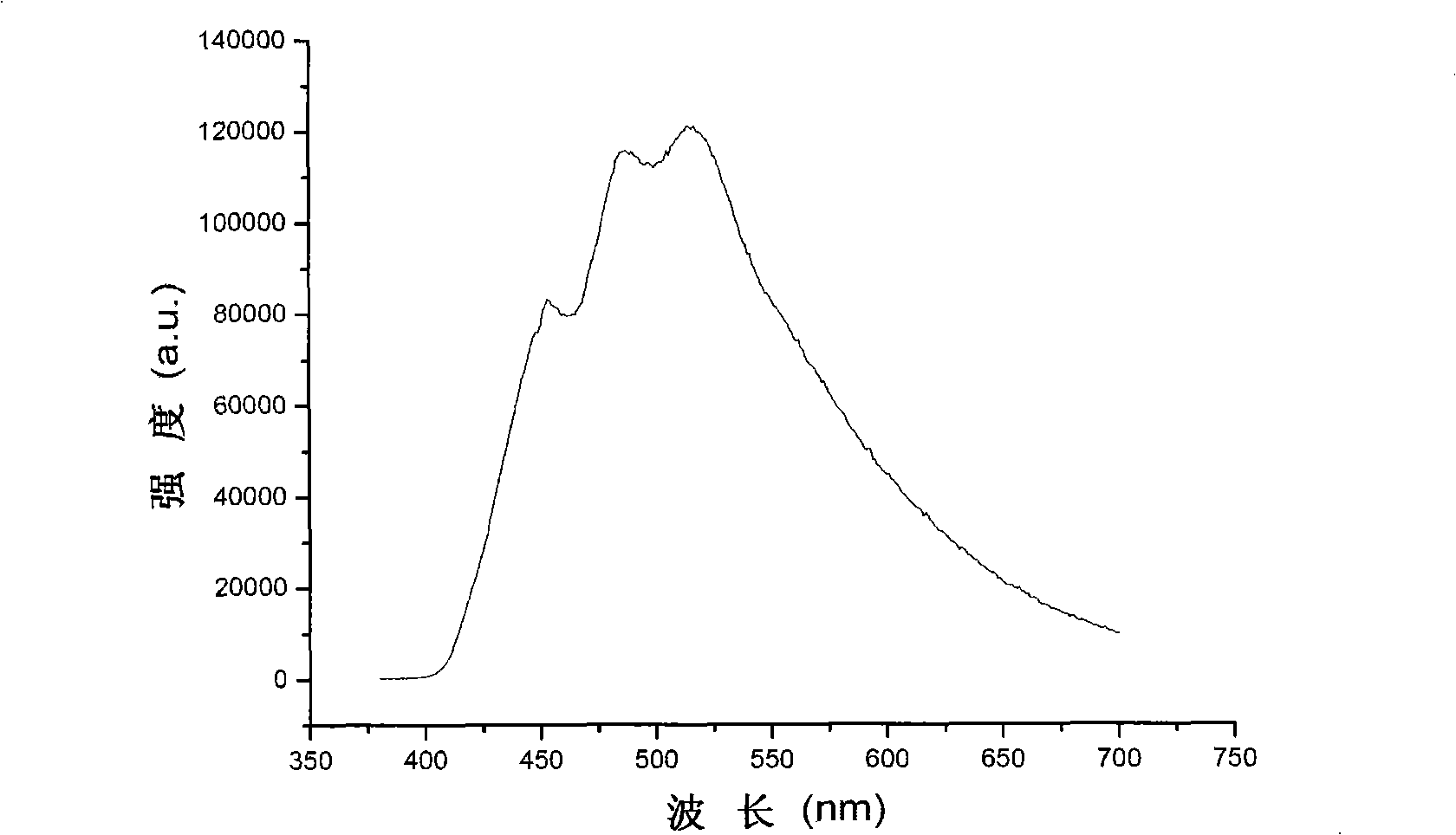
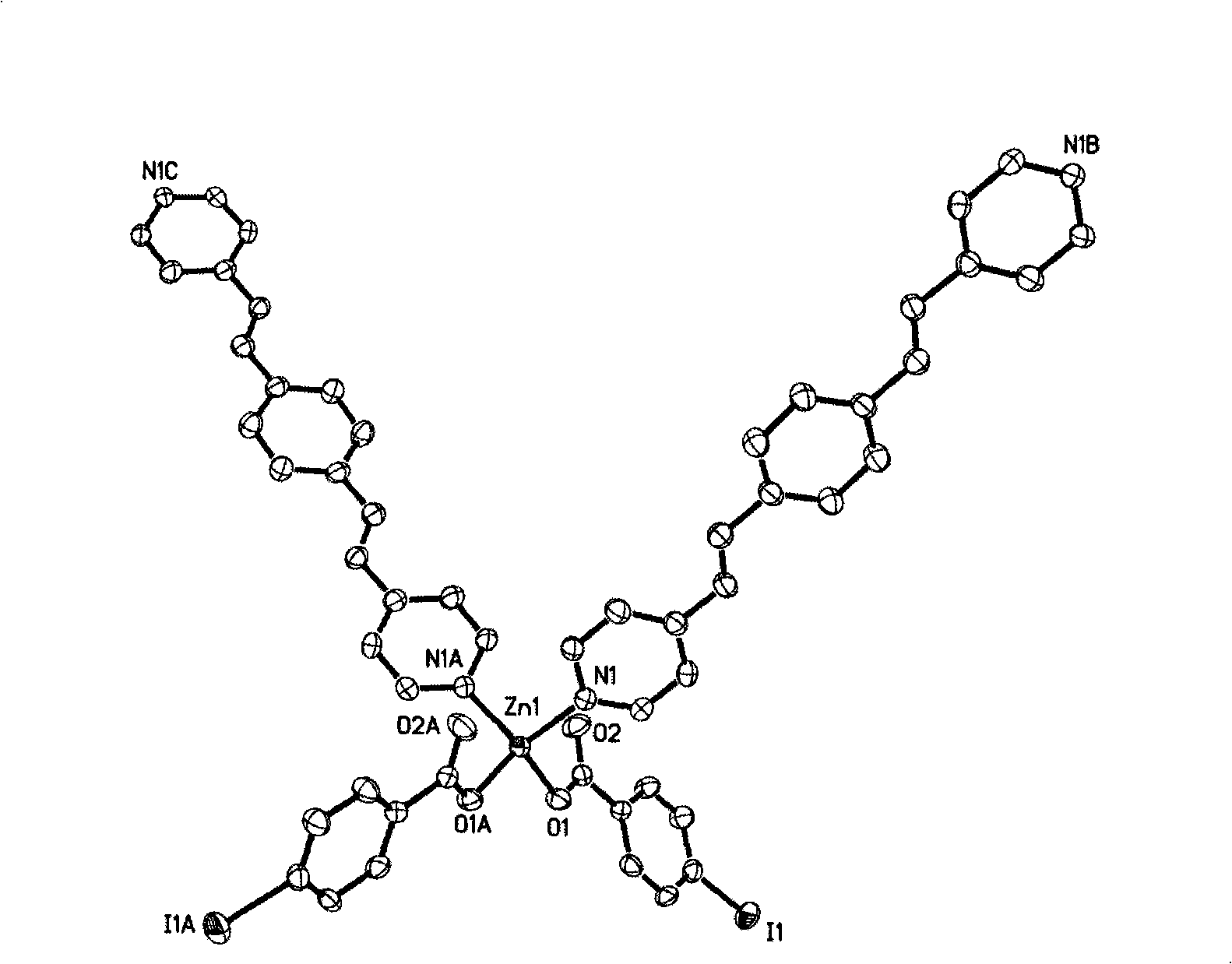
[0030] Take zinc nitrate hexahydrate (0.15g, 0.5mmol), p-iodobenzoic acid (0.25g, 1mmol) and β, β'-bis(4-pyridyl) divinylbenzene (0.14g, 0.5mmol) into the capacity In a 15mL reactor, add 10mL of water and seal it. The reactor was reacted at a constant temperature of 150-160°C for 3 days, and slowly cooled to room temperature to obtain yellow crystal 2(C 34 h 24 I 2 N 2 o 4 Zn). Excited by light with a wavelength of 304nm for 2, a strong emission peak will appear at 490nm.
[0031] Through Example 2, it can be seen that: β, β'-bis(4-pyridyl) divinylbenzene is used as a metal complex, a cluster compound and a coordination compound with good optical, magnetic, catalytic, adsorption and other properties to be synthesized with metal ions. Rigid linear ligands for polymers.
[0032] Because the coordination polymers formed by the commonly used analogues 4,4'-bipyridine and 1,2-bis(4-pyridyl)ethylene have these functions, and β,β'-bis(4-pyridyl)bis Vinylbenzene has a greater ...
Embodiment 3
[0034] Product 1 was characterized by infrared, hydrogen spectrum, elemental analysis, etc. Compound 2 was characterized by infrared and X-ray single crystal diffraction, and the fluorescence properties of 1 and 2 were studied. The specific results are as follows.
[0035] Product 1:
[0036] IR: v(KBr) / cm -13446m, 3027w, 1592s, 1547m, 1508w, 1420s, 1325w, 1281w, 1217w, 972s, 868w, 832s, 803w, 591s, 557s.
[0037] 1 H NMR (400MHz, d 6 -DMSO, 298K, TMS): δ=8.58 (s, 4H, Py-H), 7.72 (s, 4H, Ph-H), 7.63 (s, 4H, Py-H), 7.58 (s, 2H, CH =CH), 7.34(d, 2H, CH=CH);
[0038] Elemental analysis (C 20 h 16 N 2 ): theoretical value (%): C, 84.48; H, 5.67; N, 9.85;
[0039] Found value (%): C, 84.76; H, 5.59; N, 9.87.
[0040] Compound 2:
[0041] IR: v(KBr) / cm -1 3434m, 3032w, 1613s, 1558s, 1508w, 1432w, 1390m, 1356s, 1201w, 1028m, 969m, 837s, 763s, 618m, 556s, 473m.
[0042] Crystallographic parameters of compound 2 in table 1
[0043]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com