Melittin complex nanometer granule for oral dosing and preparation method thereof
A technology of melittin and complex, applied in the field of medicine, can solve the problems of low encapsulation rate and no breakthrough progress, and achieve the effects of ensuring biological activity, overcoming bottlenecks and enhancing lipophilicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Embodiment 1: the preparation of melittin-sodium lauryl sulfate complex
[0014] Take 4 mg of melittin and 2.4 mg of sodium lauryl sulfate and dissolve them in 1 ml of distilled water respectively, mix them, collect the resulting white precipitate, and freeze-dry for 12 hours to obtain a dry powder.
Embodiment 2
[0015] Embodiment 2: Preparation of melittin-sodium deoxycholate complex
[0016] Take 2.5 mg of melittin and 2 mg of sodium deoxycholate and dissolve them in 1 ml of distilled water, mix them, collect the resulting white precipitate, and freeze-dry for 12 hours to obtain a dry powder.
Embodiment 3
[0017] Embodiment 3: Preparation of melittin complex lactic acid-glycolic acid copolymer nanoparticles
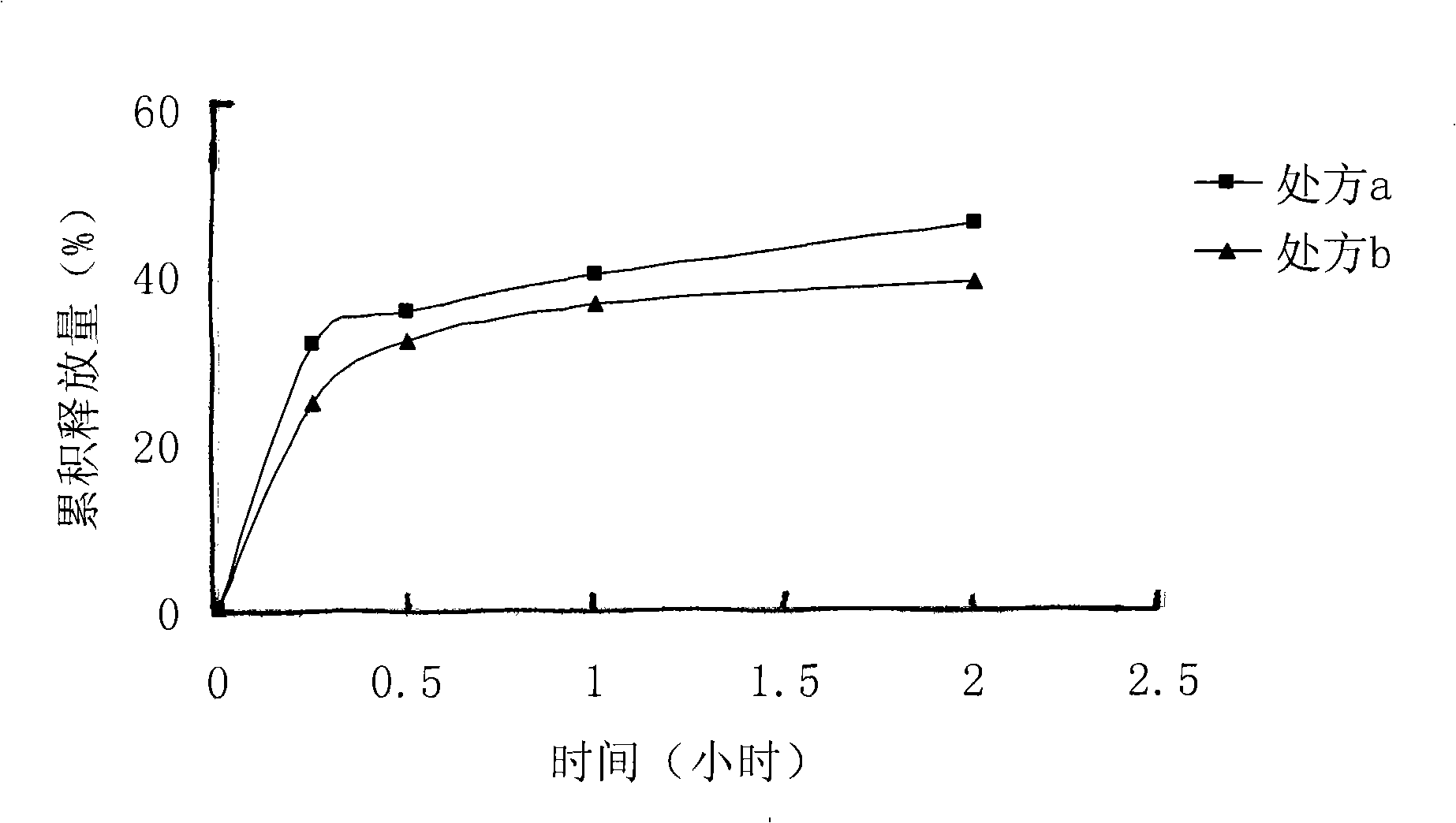
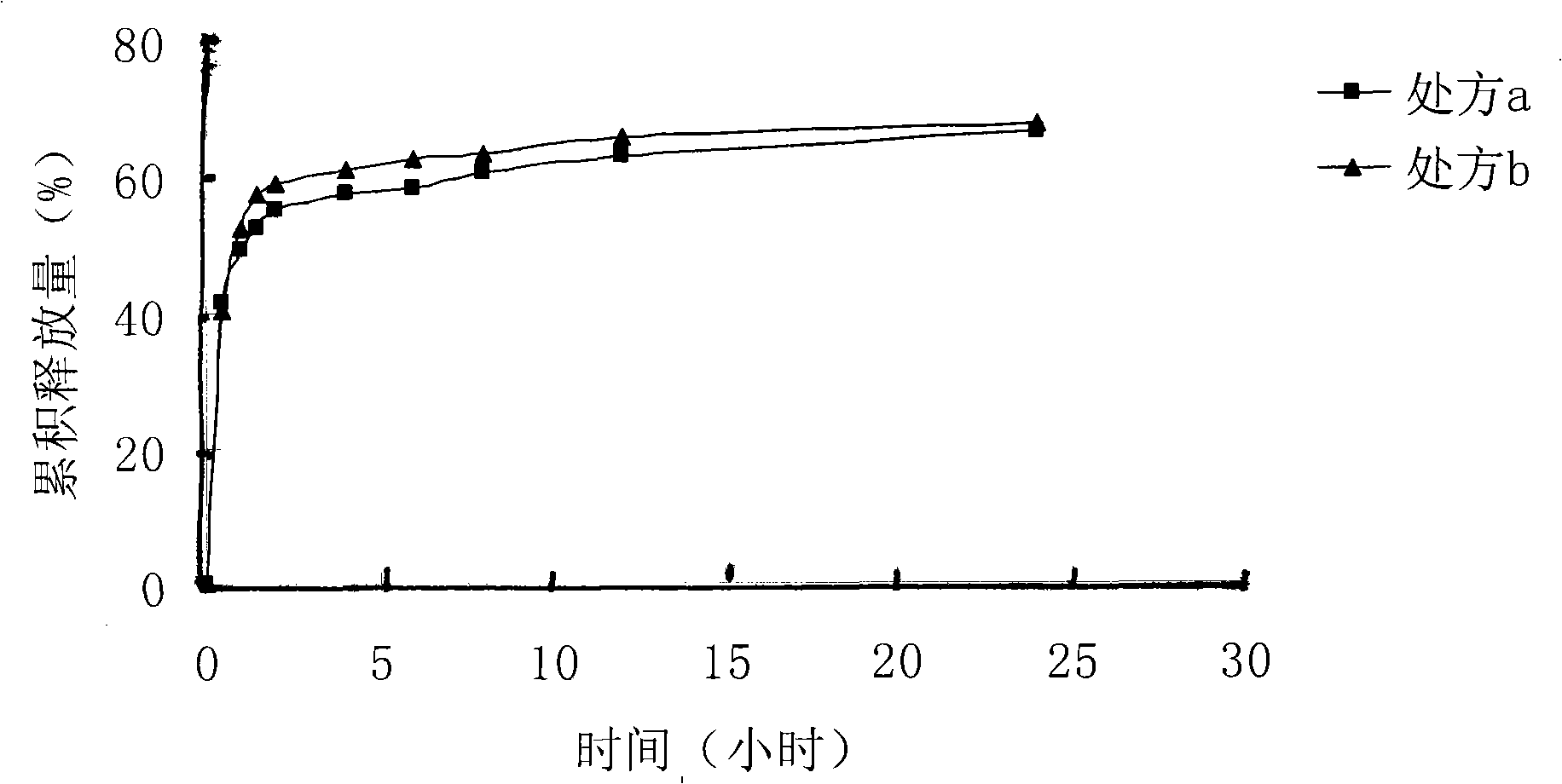
[0018] Dissolve 140 mg of lactic acid-glycolic acid (75:25) with a molecular weight of 10500 in 4 ml of dimethyl sulfoxide solvent, and stir evenly. Take 12mg of melittin-sodium lauryl sulfate complex prepared in Example 1 and add it into the polymer solution, and stir until clarification. The above solution was poured into an aqueous solution containing 1% polyvinyl alcohol, and stirred at room temperature at 500 r / min for 4 hours to obtain a blue opalescent nanosuspension. SEM pictures see figure 1 . The measured encapsulation efficiency was 96%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com