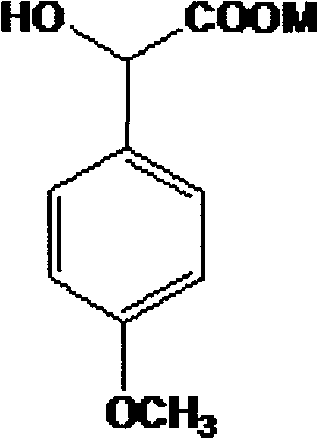
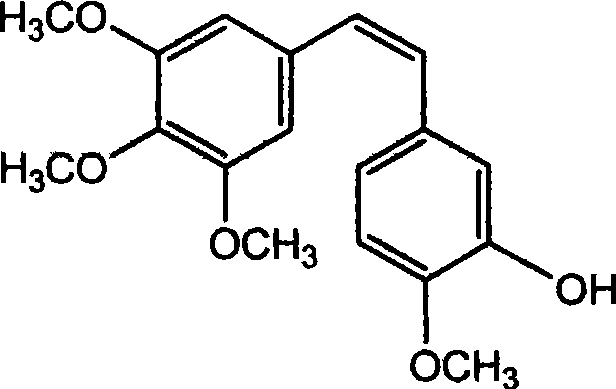
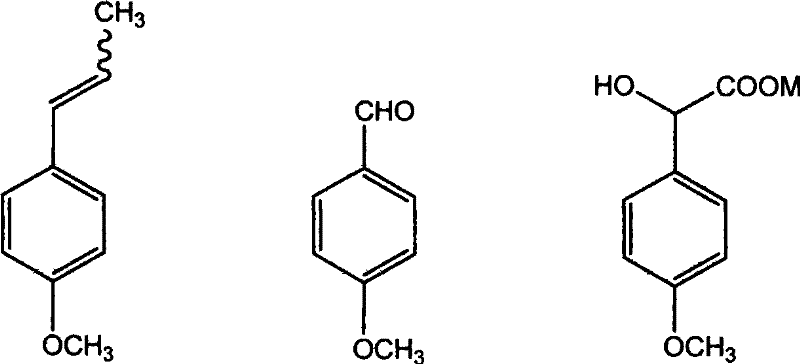
Method for preparing (Z)-3'-hydroxy-3,4,4',5-tetramethoxy diphenyl ethylene from regenerative natural plant resource
A technology of tetramethoxystilbene and natural plants, which is applied in the field of medicine and chemical industry, can solve the problems of great harm to operators and the environment, low total yield, long operation route, etc., and achieve good sustainable development ability and smooth operation. The effect of improving the counter selectivity and reducing the reaction cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Add 14.8g (0.1mol) of anethole, 8.88g of acetic acid, and 35.52g of cyclohexane in the reaction flask, stir evenly, control the reaction temperature at 20°C, and feed 2.7L (0.12mol) of O 3 , carry out ozonation reaction, 20min oxidation ends. Add NaHSO with a concentration of 200g / L in the three-necked flask 3 Aqueous solution 57mL, then slowly add ozonide dropwise, react at 80°C for 60min, cool down to 50°C, adjust to pH ≥ 10 with sodium hydroxide solution, stand and separate layers, the upper layer is the crude product of anisaldehyde, and the lower layer is the aqueous phase with cyclohexane After extracting the alkanes, add it to the crude product of anisaldehyde. The crude product of anisaldehyde is dried with anhydrous sodium sulfate and rectified under reduced pressure. p-Methoxybenzaldehyde weighs 10.88g, and the yield is 80%.
Embodiment 2
[0051] Add 14.8g (0.1mol) of anethole, 8.88g of acetic acid, and 35.52g of cyclohexane in the reaction flask, stir evenly, control the reaction temperature at 0°C, and feed 2.24L (0.10mol) of O 3 Carry out ozonation reaction, 40min oxidation finishes. Add NaHSO with a concentration of 200g / L in the three-necked flask 3 Aqueous solution 52mL, then slowly add ozonide dropwise, react at 60°C for 90min, cool down to 50°C, adjust to pH ≥ 10 with sodium hydroxide solution, stand and separate layers, the upper layer is the crude anisaldehyde, and the lower layer is the aqueous phase with cyclohexane After extracting the alkanes, add it to the crude product of anisaldehyde. The crude product of anisaldehyde is dried with anhydrous sodium sulfate and rectified under reduced pressure. p-Methoxybenzaldehyde weighs 9.79g, and the yield is 72%.
Embodiment 3
[0053] Add 14.8g (0.1mol) of anethole and 44.4g of ethanol in the reaction flask, stir well, control the reaction temperature at 0°C, adjust the ozone flow, and then feed 4.48L (0.20mol) of O 3 Carry out ozonation reaction, 40min oxidation finishes. Add concentration of 200g / L Na in the three-necked flask 2 SO 3 Aqueous solution 57mL, then slowly add ozonide dropwise, react at 60°C for 90min, cool down to 50°C, adjust to pH ≥ 10 with sodium hydroxide solution, stand and separate layers, the upper layer is the crude product of anisaldehyde, and the lower layer is the aqueous phase with cyclohexane After extracting the alkanes, add it to the crude product of anisaldehyde. The crude product of anisaldehyde is dried with anhydrous sodium sulfate and rectified under reduced pressure. p-Methoxybenzaldehyde weighs 9.52g, and the yield is 70%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com