Ultra-fine dry powder particle suitable for drug administration for lung, and preparation method thereof
A dry powder and granule technology, which can be applied to pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc. It can solve the problems of low particle deposition rate, inconvenient practical application, and easy inactivation of active pharmaceutical ingredients.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0126] Example 1. Levodopa (Levodopa) dry powder preparation
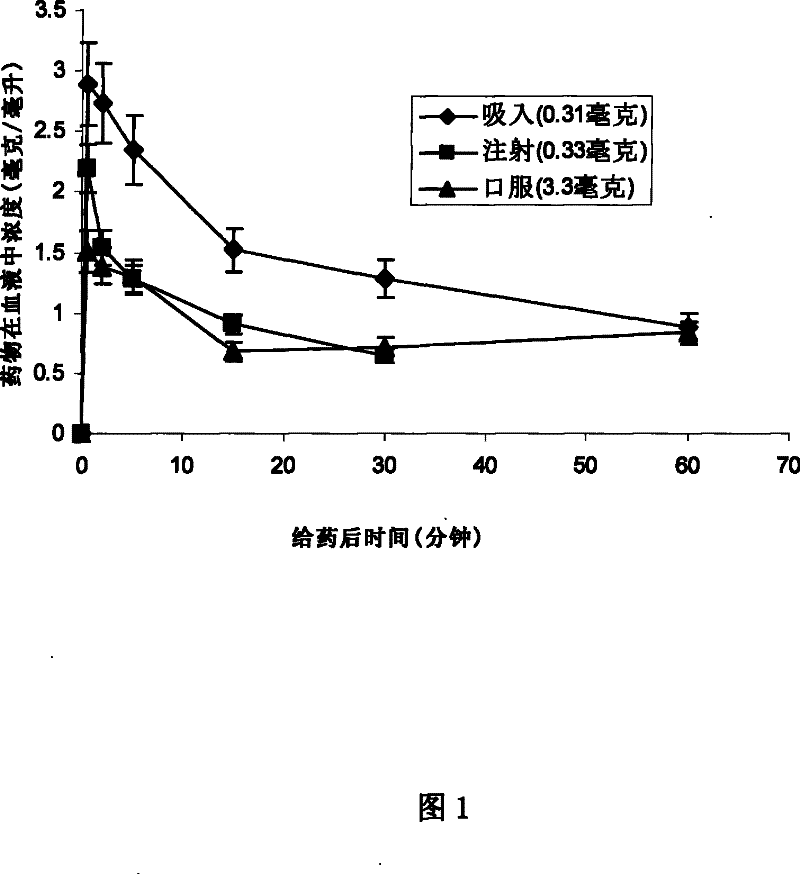
[0127] Parkinson's disease is an age-related degenerative disorder of the central nervous system, the symptoms of which can be manifested as inactivity, slowness, rigidity of movement and tremor. Oral drug levodopa has a significant effect on early Parkinson's hypokinetic symptoms. However, the current oral dopa drugs have other shortcomings. With the development of the disease, the efficacy of the drug is limited, and the bioavailability of the drug is difficult. It is predicted that these defects caused by oral administration may be completely eliminated by pulmonary inhalation administration.
[0128] An inhalable dry powder containing levodopa can be made in the following manner. In this example, the granules contain 20% (by weight, the same below) of levodopa, 20% of sodium citrate, 10% of calcium chloride, And 50% 1,2-dipalmitoyl-sn-propanetriyl-3-phosphatidylcholine, i.e. Dppc.250 milliliters, the formula s...
example 2
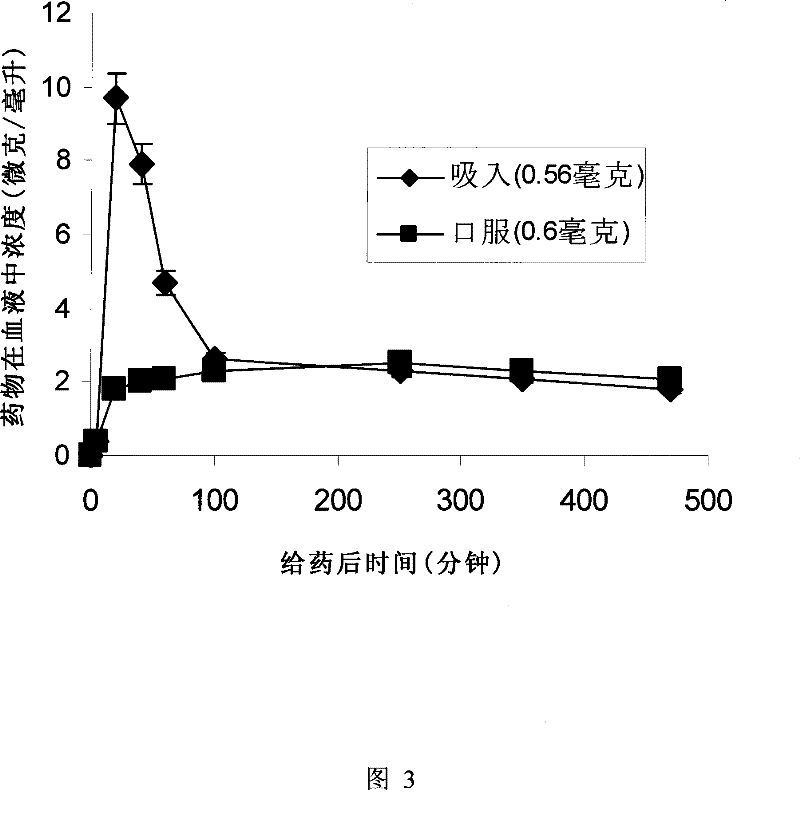
[0136] Example 2. Ketoprofen dry powder preparation
[0137] Ketoprofen is a non-steroidal anti-inflammatory drug approved by the US Food and Drug Administration. Pain-relieving drugs, as commonly used drugs in daily life and medical treatment, have huge market potential, and at the same time, there is an urgent need to improve the curative effect of existing drugs. As we all know, whether it is treating acute pain or penetrating pain, it is very important to make the drug concentration in the blood reach the effective therapeutic level as soon as possible so as to relieve pain. According to the characteristics of the inhalation administration method, the inhalation administration of analgesic drugs will have unique advantages in terms of rapid onset of effect. There is no doubt that there will be benefits for patients.
[0138] Inhalable dry powder containing ketoprofen (Ketoprofen) can be prepared as follows, in this example, the granule contains 20% (by weight) of ketopro...
example 3
[0144] Example 3. Epinephrine dry powder preparation
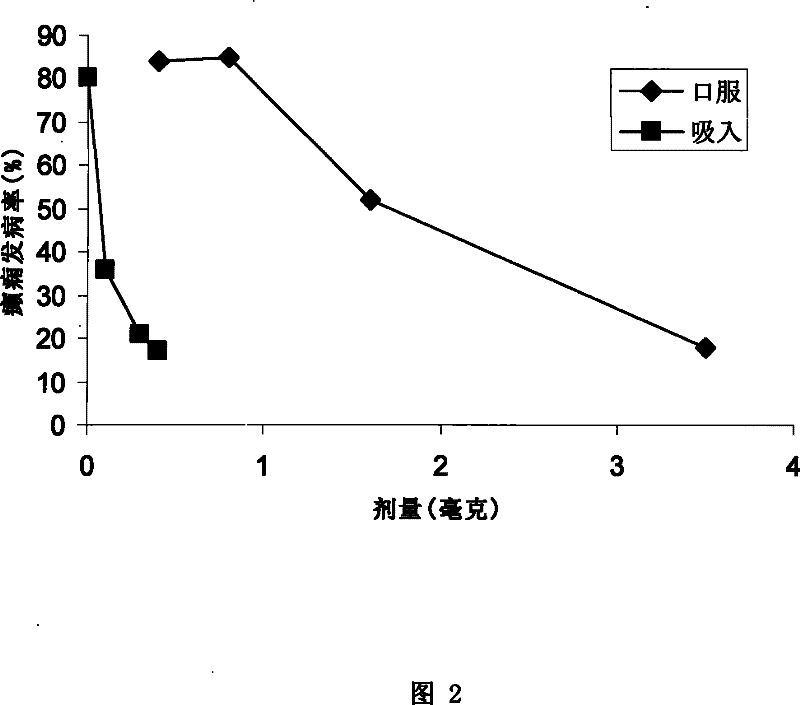
[0145] Anaphylaxis and death from anaphylaxis are becoming more common, especially in children and young adults, and the rapid onset and severity of symptoms make the treatment of anaphylaxis extremely difficult.
[0146] Intramuscular injection of adrenaline is the most important drug in the treatment of allergies, and it is very effective, but many allergy medicine experts worry about serious consequences and even death caused by delay in administration due to pain caused by intramuscular injection. The inhalation type can completely change the user's fear of needles, and can make the medicine quickly enter the blood to act on the disease.
[0147] Dry powders containing epinephrine can be prepared as follows. In this example, the dry powder granules contain 10% epinephrine (percentage by weight, the same below). 60% DPPC, 20% sodium citrate and 10% calcium chloride. 250 milliliters of solid concentration is 3 grams p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com