Phenolphthalein type cyanate monomer, polymeric compounds and methods of formulating same
A technology of phenolphthalein-type cyanate and cyanate, applied in the direction of organic chemistry, can solve the problems of reducing key physical properties, no cyanate compounds containing phenolphthalein structure, etc., and achieve good thermal stability and high glass transition temperature , the effect of low water absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Synthesis and curing of phenolphthalein cyanate: Dissolve phenolphthalein (2.387g, 0.0106mol) in 40ml of acetone, cool to -10°C, add cyanogen bromide (2.595g, 0.0245mol) and triethylamine (3ml, 0.02152mol ), and continue to insulate and stir for 0.5 hours to terminate the reaction.
[0030] The above mixture was filtered, the filtrate was concentrated under reduced pressure, poured into a large amount of n-hexane to settle, filtered to obtain the crude product, recrystallized in ether to obtain white crystals, and dried to obtain cyanate ester 3.86g, yield 91%, mp: 135.4°C.
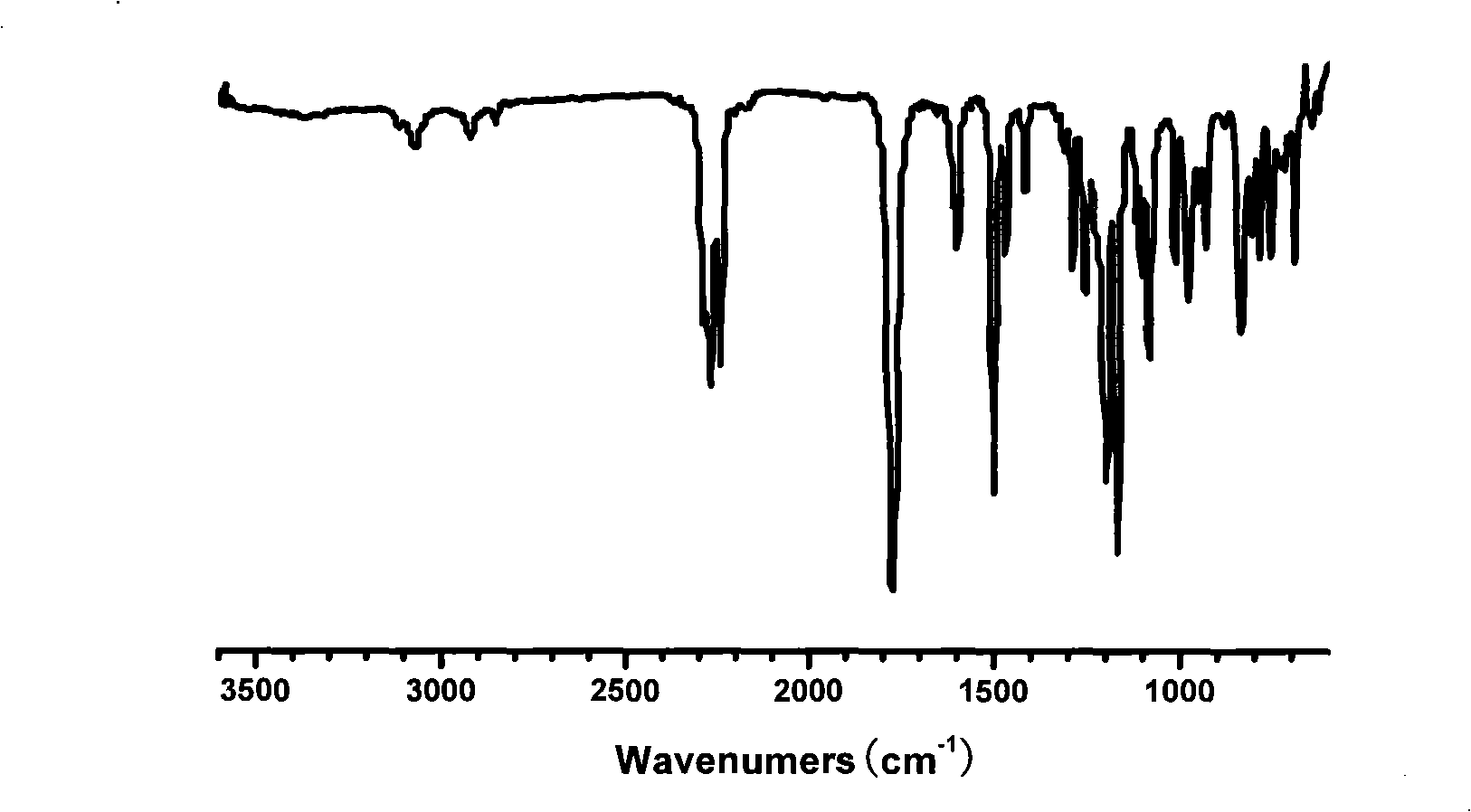
[0031] Infrared spectrum display (see figure 1 ), at 2239, 2268cm -1 A strong infrared absorption peak appears at , which is the characteristic absorption peak of the cyano group, and at the same time 3373cm -1 The vibration peak of the phenolphthalein hydroxyl group becomes very gentle, indicating that the reaction is relatively complete.
[0032] After the cyanate compound prepared above was ...
Embodiment 2
[0035] Synthesis and solidification of o-cresol phthalocyanate: dissolve o-cresol phthalein (3.689g, 0.0106mol) in 40ml of chloroform, cool to -20°C, add cyanogen bromide (2.595g, 0.0245mol) and triethylamine ( 3ml, 0.02152mol), the reaction was terminated after stirring for 120 minutes.
[0036] The above mixture was filtered, poured into a large amount of petroleum ether for sedimentation, recrystallized with ethyl acetate, and filtered to obtain 4.04 g of cyanate, with a yield of 89%, and mp: 146.8°C.
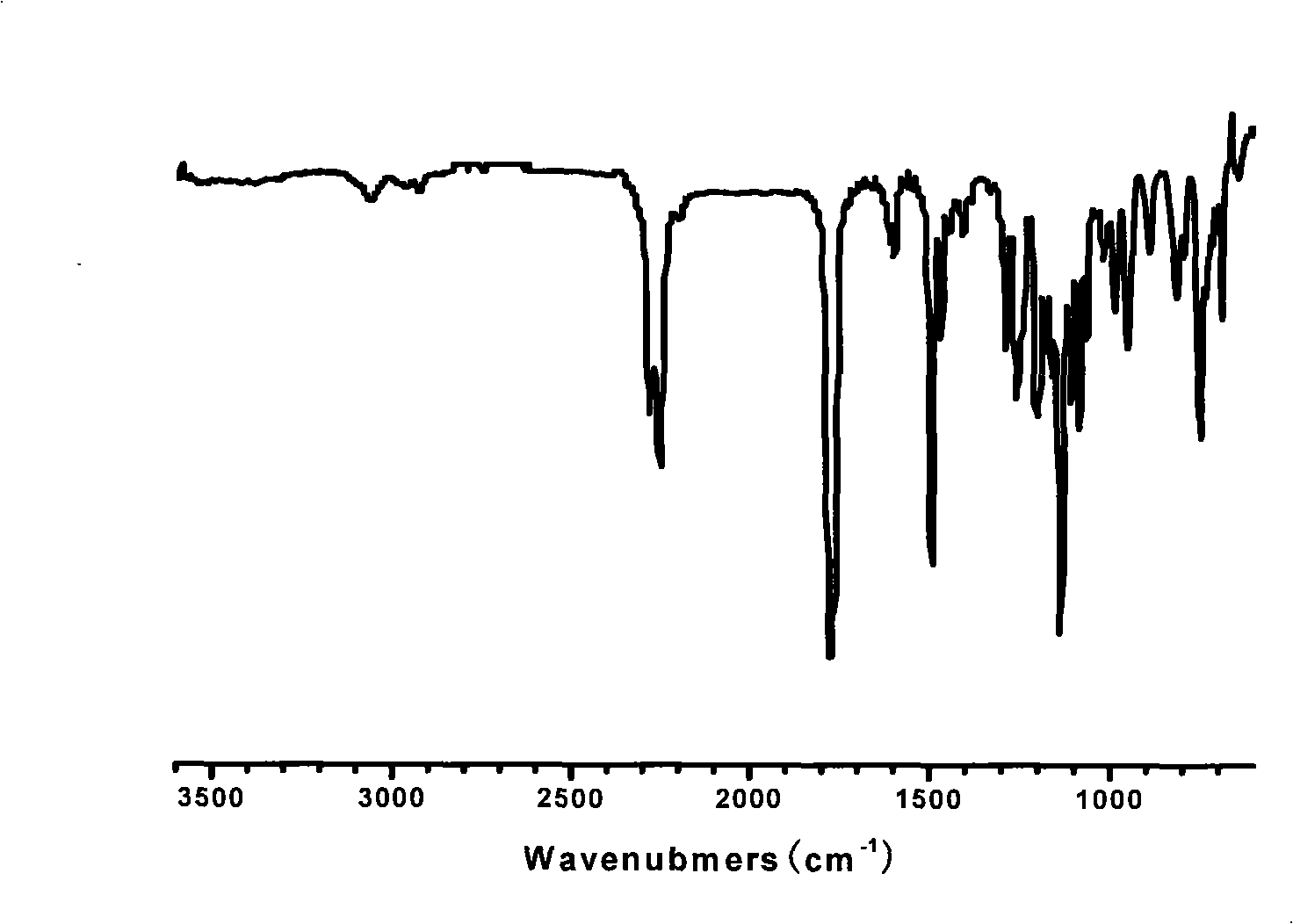
[0037] Infrared spectrum display (see image 3 ), at 2253, 2275cm -1 A strong infrared absorption peak appears at , which is the characteristic absorption peak of cyano group, and at the same time 3412cm -1 The vibration peak of the o-cresolphthalein hydroxyl group becomes very gentle, indicating that the reaction is relatively complete.
[0038] After the cyanate ester monomer prepared above was degassed at 170°C for 1h, it was cured according to the temperature rise pro...
Embodiment 3
[0041] Synthesis and solidification of thymolphthalein cyanate: dissolve thymolphthalein (3.057g, 0.0071mol) in 40ml toluene, cool to -5°C, add cyanogen bromide (1.729g, 0.0163mol) and triethylamine ( 2ml, 0.0071mol), stirred for 4 hours to terminate the reaction.
[0042] The above mixture was filtered through a large amount of cyclohexane to obtain a crude product, which was recrystallized with petroleum ether at 60-90°C, filtered and dried to obtain 3.34 g of cyanate, with a yield of 92%, and mp: 196°C.
[0043] Infrared spectrum display (see Figure 5 ), at 2231, 2263cm -1 A strong infrared absorption peak appears at , which is the characteristic absorption peak of the cyano group, and at the same time 3403cm -1 The vibration peak of the phenolphthalein hydroxyl group becomes very gentle, indicating that the reaction is relatively complete.
[0044] After the cyanate compound prepared above was degassed at 200°C for 1h, it was cured according to the heating program of 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com