Lansoprazole enteric coated tablet and preparing method thereof
A technology of lansoprazole enteric and lansoprazole is applied in the field of lansoprazole enteric-coated tablets and its preparation, which can solve the problems of inappropriate large-scale mechanized production, complicated manufacturing process and high production cost, and achieve dissolution rate and The effect of good bioavailability, simple preparation method, and small difference in drug content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
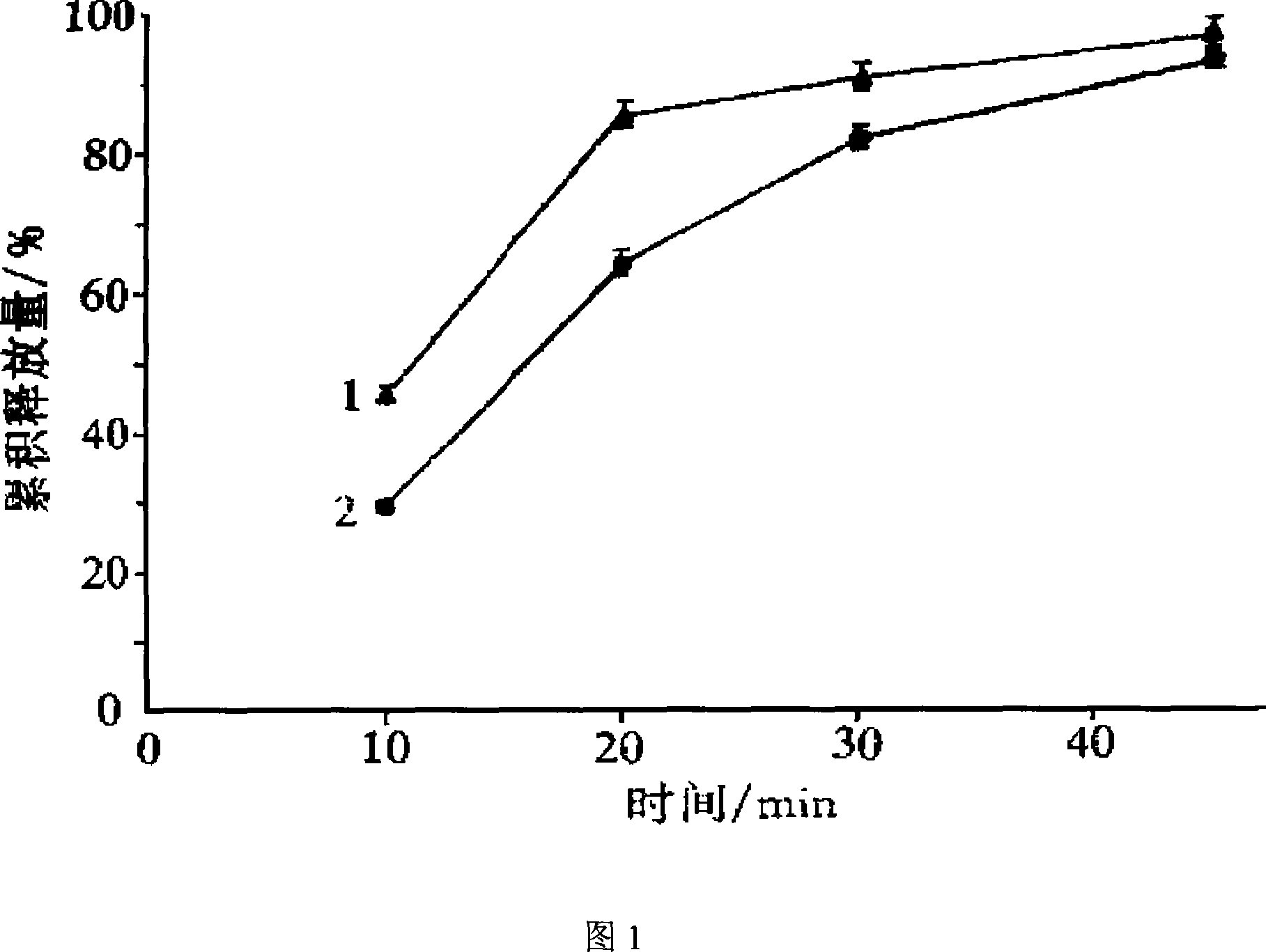
Image
Examples
Embodiment 1
[0057] Prescription (make 1000 tablets):
[0058] (1) Tablet core composed of lansoprazole and pharmaceutically acceptable excipients
[0059] Lansoprazole 15g
[0060] Starch 90g
[0061] Microcrystalline Cellulose 20g
[0062] Hypromellose 10g
[0063] Proper amount of ethanol
[0064] Carboxymethyl starch sodium 8g
[0065] Magnesium Stearate Appropriate amount
[0066] (2) Enteric-coated layer
[0067] Polyacrylic resin No. II 5g
[0068] Diethyl phthalate 2ml
[0069] Castor oil 1ml
[0070] Tween-80 2ml
[0071] Ethanol 90ml
[0072] Preparation:
[0073] 1) Preparation of tablet core
[0074] Get the prescription amount of Lansoprazole, microcrystalline cellulose, hydroxypropyl cellulose, pulverize and sieve, mix, add binder ethanol, mix and granulate, dry, 16 mesh sieve granulate, add appropriate amount, carboxymethyl starch sodium, Magnesium stearate, compressed into tablets, that is, Lansoprazole tablet core;
[0075] 2) Preparation of Enteric Layer Co...
Embodiment 2
[0080] Prescription (make 1000 tablets):
[0081] (1) Tablet core composed of lansoprazole and pharmaceutically acceptable excipients
[0082] Lansoprazole 15g
[0083] Starch 110g
[0084] Microcrystalline Cellulose 10g
[0085] Proper amount of ethanol
[0086] Magnesium Stearate Appropriate amount
[0087] (2) Enteric-coated layer
[0088] Polyacrylic resin No. III 5g
[0089] Diethyl phthalate 2ml
[0090] Castor oil 1ml
[0091] Tween-80 2ml
[0092] Ethanol 90ml
[0093] Preparation:
[0094] 1) Preparation of tablet core
[0095] Get the prescription amount of lansoprazole, starch, microcrystalline cellulose, pulverize and sieve, mix, add binder ethanol, mix and granulate, dry, 16 mesh sieve granulate, add appropriate amount of magnesium stearate, tabletting, namely Delansoprazole tablet core;
[0096] 2) Preparation of Enteric Layer Coating
[0097] Dissolve polyacrylic acid resin No. II in ethanol, add prescription amount of diethyl phthalate, Tween-80 a...
Embodiment 3
[0101] Prescription (make 1000 tablets):
[0102] (1) Tablet core composed of lansoprazole and pharmaceutically acceptable excipients
[0103] Lansoprazole 15g
[0104] Starch 90g
[0105] Microcrystalline Cellulose 20g
[0106] Hypromellose 10g
[0107] Proper amount of ethanol
[0108] Carboxymethyl Starch Sodium 3g
[0109] Magnesium Stearate Appropriate amount
[0110] (2) Enteric-coated layer
[0111] Polyacrylic resin No. II 5g
[0112] Diethyl phthalate 2ml
[0113] Castor oil 1ml
[0114] Tween-80 2ml
[0115] Ethanol 90ml
[0116] Preparation:
[0117] 1) Preparation of tablet core
[0118] Take the prescription amount of lansoprazole, starch, microcrystalline cellulose, and hydroxypropyl cellulose, pulverize and sieve, mix, add binder ethanol, mix and granulate, dry, granulate with a 16-mesh sieve, add an appropriate amount of sodium carboxymethyl starch , magnesium stearate, compressed into tablets, that is, lansoprazole tablet core;
[0119] 2) Prepa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com