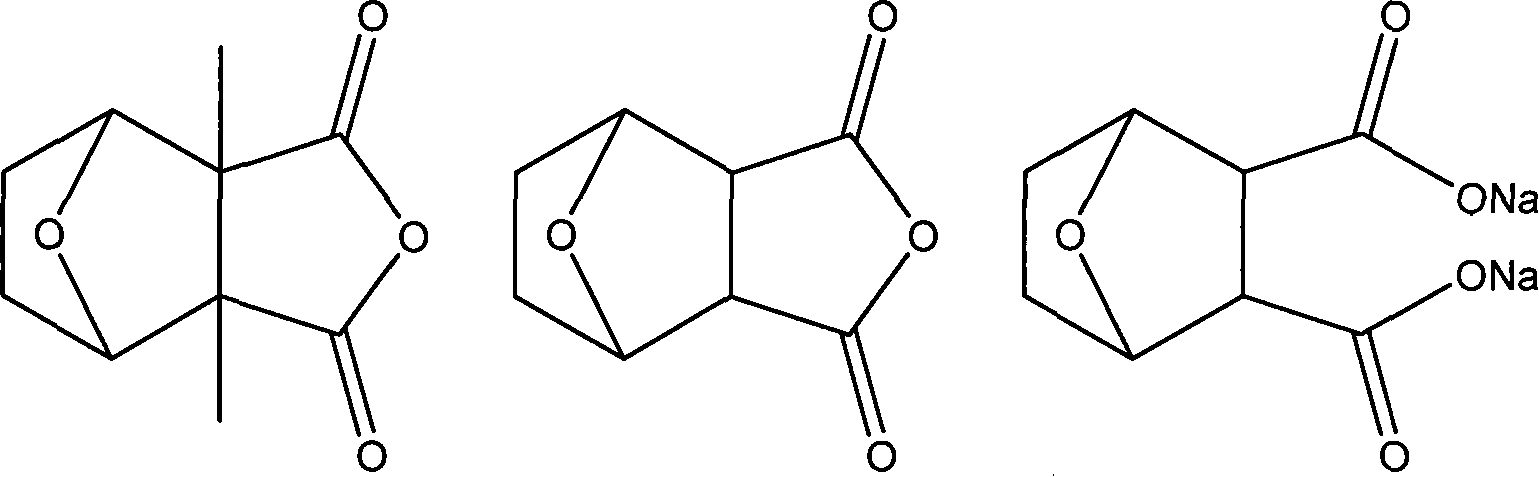
Injectio natarii norcantharidatis freeze-dried powder for injection and preparing method thereof
A technology of sodium demethylcantharidinate and sodium cantharidinate, which is applied in the field of medicine, can solve problems such as irrationality of freeze-drying curves, difficult removal of water, and shortened storage period, so as to ensure drug safety, good shape and appearance, and improve The effect of product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Add 5 g of sodium hydroxide to 1500 ml of water for injection to prepare a solution, add 10 g of norcantharidin and 100 g of mannitol, stir to dissolve, adjust the pH to 9.1 to 9.5 with hydrochloric acid or sodium hydroxide, add water for injection until the volume of the solution is 2000 ml, and add to the Add 0.01% medical activated carbon to the prepared solution, stir at room temperature for 20 minutes, filter to remove carbon, and then fine filter the filtrate through a 0.22 μm microporous filter membrane. 57°C, the cooling rate is 17°C / min, heat preservation and freezing for 3 hours, vacuumize the pre-frozen medicinal solution to 10Pa, then slowly and uniformly heat up to 0°C at a speed of 3.2°C / hour, and keep warm for 3 hours, sublimation The finished drug is heated to 25°C at a uniform speed, the heating time is 4 hours, and then heat-preserved and dried for 5 hours, tested, pressed and packed, and packed into the warehouse.
Embodiment 2
[0042] Add 5 g of sodium hydroxide to 1500 ml of water for injection to prepare a solution, add 10 g of norcantharidin and 100 g of mannitol, stir to dissolve, adjust the pH to 9.3 with hydrochloric acid or potassium hydroxide, add water for injection until the volume of the solution is 2000 ml, and add to the prepared solution Add 0.01% medical activated carbon, stir at room temperature for 20 minutes, filter to remove carbon, and then filter the filtrate through a 0.22μm microporous filter membrane. After the fine filter filtrate is tested and qualified, it will be subpackaged, and the subpackaged drug solution will be quickly cooled to -60°C , the cooling rate is 25°C / min, heat preservation and freezing for 3 hours, vacuumize the pre-frozen medicinal solution to 15Pa, then slowly and uniformly heat up to -5°C at a speed of 3°C / hour, and keep warm for 5 hours, and the sublimation is completed The drug is heated to 20°C at a uniform speed, the heating time is 5 hours, and then...
Embodiment 3
[0044] Add 5g of sodium hydroxide to 1500ml of water for injection to prepare a solution, add 10g of norcantharidin and 100g of mannitol, stir to dissolve, adjust the pH to 9.5 with hydrochloric acid or sodium hydroxide, add water for injection until the volume of the solution is 2000ml, add to the prepared solution Add 0.01% medical activated carbon to the solution, stir at room temperature for 20 minutes, filter to remove carbon, and then filter the filtrate through a 0.22μm microporous membrane. After the fine filter filtrate passes the test, it will be subpackaged, and the subpackaged drug solution will be quickly cooled to -55°C. , the cooling rate is 15°C / min, heat preservation and freezing for 3 hours, vacuumize the pre-frozen medicinal solution to 5Pa, then slowly and uniformly heat up to 5°C at a speed of 3.5°C / hour, keep warm for 2 hours, and sublimate the liquid The medicine is heated to 30°C at a uniform speed, and the heating time is 3 hours, and then it is kept wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com