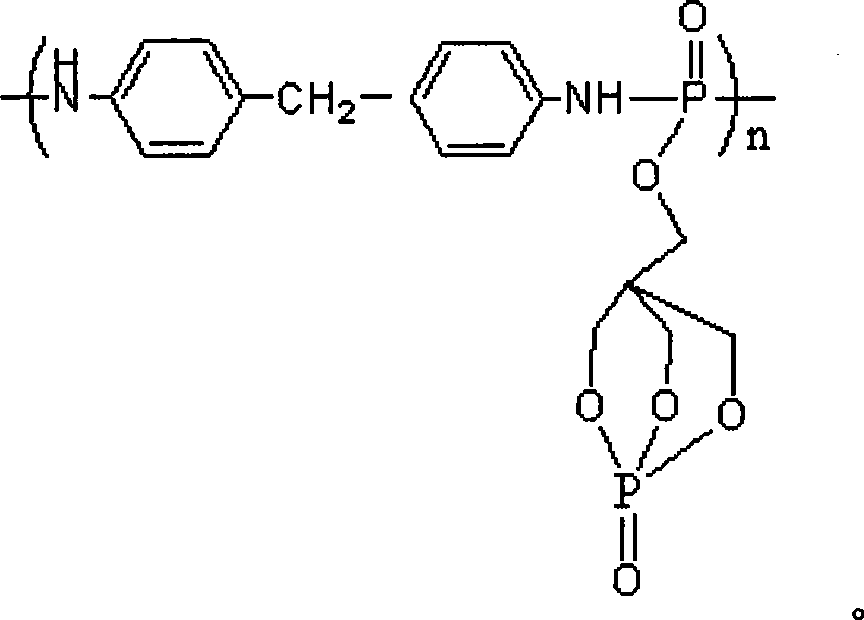
Buckling flame retardant containing phosphor-nitrogen macromolecule and preparation method thereof
An intumescent flame retardant and macromolecular technology, applied in fire-resistant coatings and other directions, can solve the problems of easy migration or precipitation, loss of flame retardant performance, small molecular weight, etc., and achieve good application prospects, easy control, and simple preparation methods. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
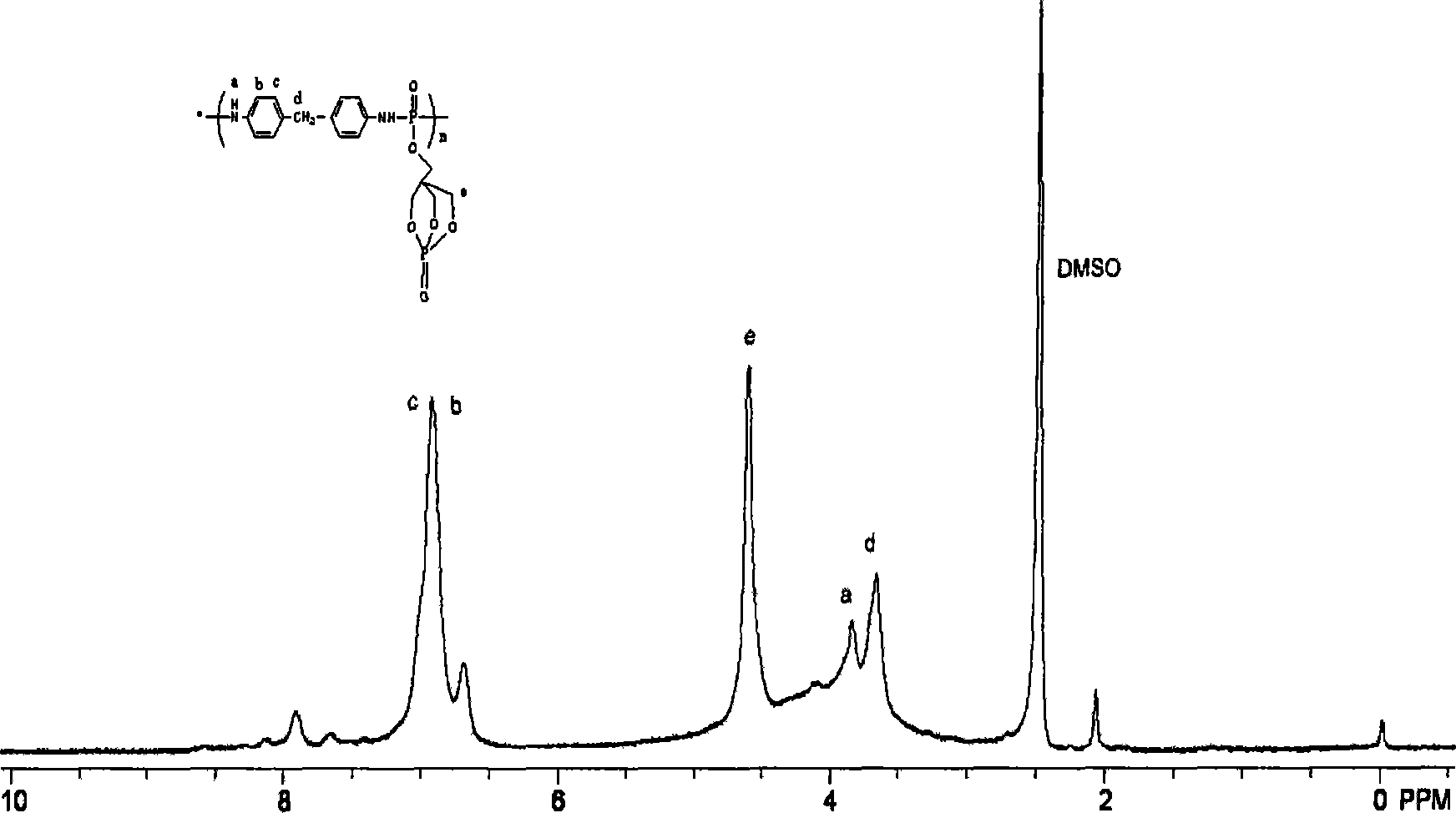
[0026] Under nitrogen protection, 59.4g of 2,6,7-trioxo-1-phosphabicyclo-[2,2,2]-octane-1-methoxy dichlorophosphate was dissolved in 100ml of acetonitrile In a 250ml three-neck flask, heat while stirring. After the dissolution is complete, start to drop 40g of 4,4'-diaminodiphenylmethane in acetonitrile solution. After the dropwise addition, start to drop 15ml of catalyst pyridine. During the reaction, light yellow powder was continuously generated, reacted for 5 hours, filtered, washed twice with deionized water, and then washed twice with absolute ethanol. Dry in a vacuum oven at 80° C. for 12 hours to obtain a light yellow powder solid with a yield of 85%. Melting point 255-258°C, elemental analysis: C%: 48.1%, H%: 5.0%, N%: 4.83%, O%: 25.8%, P%: 16.27%. See Figure 1 for the NMR spectrum. The result proves that the compound synthesized by the preparation method of the present invention is basically consistent with the designed phosphorus- and nitrogen-containing macromole...
Embodiment 2
[0030] Under nitrogen protection, 29.7g of 2,6,7-trioxo-1-phosphabicyclo-[2,2,2]-octane-1-methoxy dichlorophosphate was dissolved in 80ml of acetonitrile In a 250ml three-neck flask, heat while stirring, and after it dissolves, start to drop 20g of 4,4'-diaminodiphenylmethane in acetonitrile solution, after the dropwise addition, start to drop 10ml of catalyst pyridine, at 80°C During the reaction, light yellow powder was continuously generated. After 2 hours of reaction, it was filtered, and the filter cake was washed twice with deionized water and then twice with absolute ethanol. After drying in a vacuum oven at 80°C for 12 hours, a light yellow powder solid was obtained with a yield of 75%. Melting point 255-257°C, elemental analysis: C%: 46.2%, H%: 4.8%, N%: 5.85%, O%: 25.9%, P%: 17.25%.
Embodiment 3
[0032] Under nitrogen protection, 59.4g of 2,6,7-trioxo-1-phosphabicyclo-[2,2,2]-octane-1-methoxy dichlorophosphate was dissolved in 120ml of acetonitrile In a 250ml three-neck flask, heat while stirring. After the dissolution is complete, start to drop 42g of 4,4'-diaminodiphenylsulfone in acetonitrile solution. After the dropwise addition, start to add 20ml of catalyst pyridine dropwise. During the reaction, light yellow powder was continuously generated, reacted for 4 hours, filtered, washed twice with deionized water, and then washed twice with absolute ethanol. Dry in a vacuum oven at 80° C. for 12 hours to obtain a light yellow powder solid with a yield of 78%. Melting point 256-258°C, elemental analysis: C%: 43.6%, H%: 4.1%, N%: 5.23%, O%: 26.9%, P%: 16.77%, S%: 3.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com