Novel method for synthesizing L-carnosine
A new method and technology of carnosine, applied in the new synthesis field of L-carnosine, can solve the problems of reducing product yield, influence of product purity, toxicity of L-carnosine, etc., achieving the effect of low toxicity and side effects and meeting the requirements of medical consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
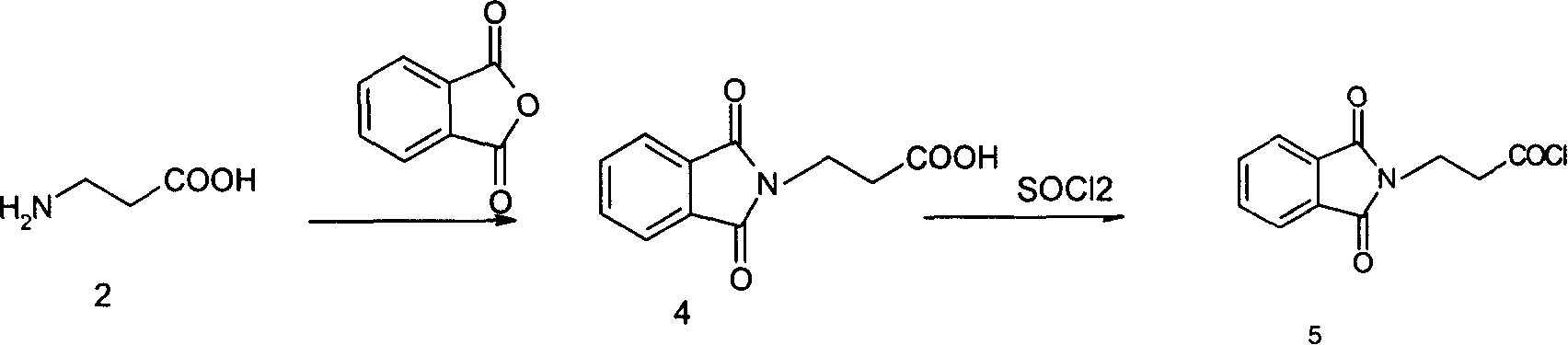
[0016] The preparation of embodiment 1 phthalyl β-alanine (4)
[0017] In 500ml there-necked flask, drop into 111g (0.75mol) phthalic anhydride, β-alanine (2) 67g (0.75mol), heat up, along with the temperature rise of reaction material, material melts slowly into liquid state, simultaneously Start stirring, and when the temperature in the kettle reaches 175° C., control the temperature and keep warm for 25 minutes. After the heat preservation is completed, the material is poured into a crystallization dish and cooled naturally to obtain 164 g of white waxy crystals, with a yield of 99.8%.
Embodiment 2
[0018] The preparation of embodiment 2 phthaloyl β-alanyl chloride (5)
[0019] Put 1200g of toluene and 151g (0.69mol) of phthalyl β-alanine (4) into a 2000ml three-necked flask, stir in a water bath to raise the temperature, control the temperature below 60°C, and add 88.0g (0.74mol) of thionyl chloride dropwise. ), kept at 60-65°C for about 3 hours (making the system transparent), after the insulation was completed, concentrated toluene under reduced pressure to dryness, then added fresh toluene to entrain thionyl chloride, and dried to obtain 162g. The yield was 99%, add 800g chloroform, dissolve white phthaloyl β-alanyl chloride (5) and prepare for the next reaction.
Embodiment 3
[0020] Embodiment 3 Preparation of phthaloyl β-alanyl-L-histidine hydrochloride (6)
[0021] Put 92g (0.59mol) of L-histidine and 256.4g (2.36mol) of trimethylchlorosilane into a 2000ml nitrogen-protected flask, stir, and feed nitrogen at the same time to make it react under anoxic and anhydrous state. At the same time, turn on the heating to make it warm up and reflux, keep warm under the reflux state, absorb the released hydrogen chloride with water, wait for the reaction solution to become a homogeneous transparent solution, then stop the reaction, and concentrate under reduced pressure to dryness (control the temperature of the water bath to be no more than 50°C ), then add 200g toluene for 2-3 times to carry out entrainment, so that the reaction solution does not contain trimethylchlorosilane raw material, after entrainment is complete, drop to normal temperature to obtain intermediate (8); add 260g chloroform, and cool to 0-5 ℃, dropwise add the chloroformyl chloride (5)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com