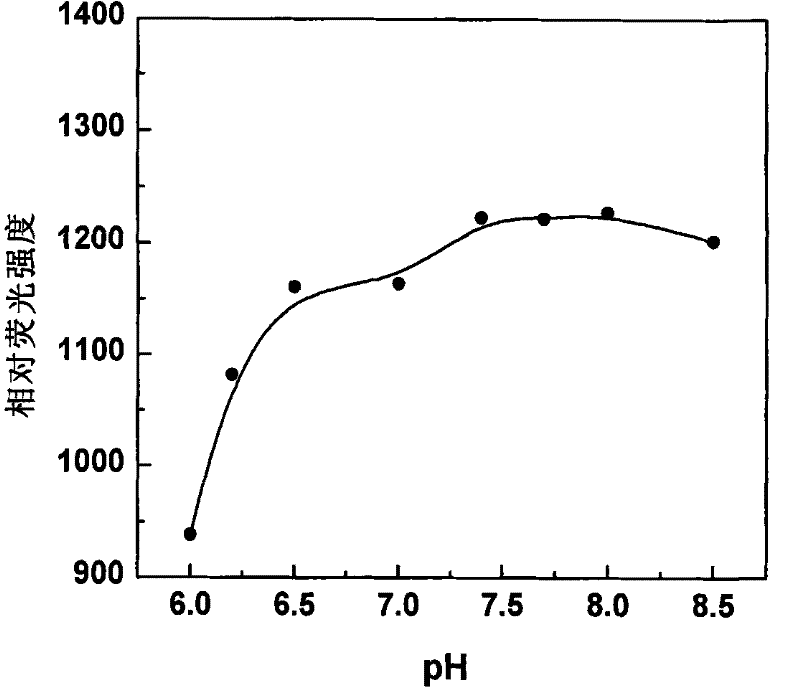
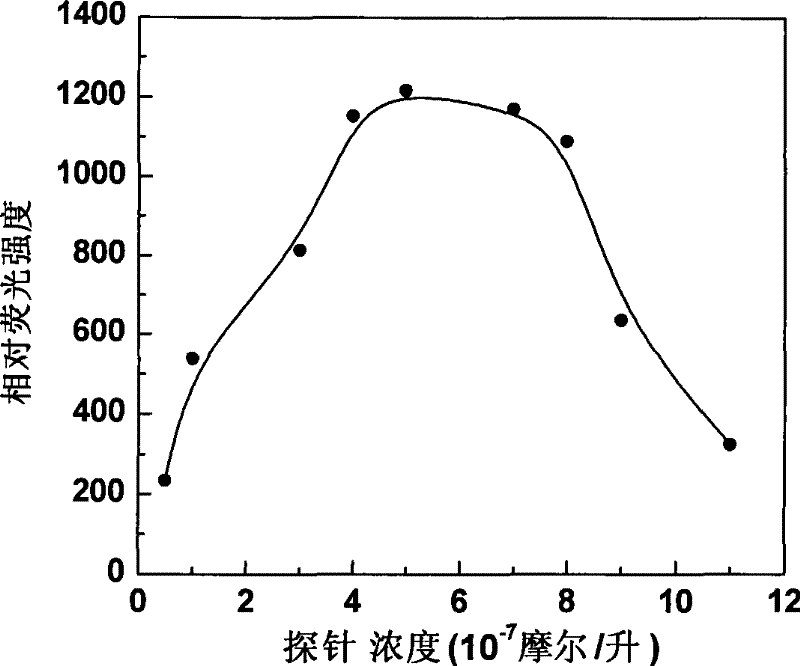
Fluorescent probe in use for detecting glutathion inside cell, synthesis method and application
A fluorescent probe, glutathione technology, applied in the field of biological detection and clinical medical detection, can solve the problems of unsatisfactory selectivity and achieve good selectivity, easy large-scale production, and stable performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] a. Dissolve 150 mg of potassium selenide in 10 ml of N,N-dimethylformamide, then add 250 mg of o-trifluoromethylbromobenzene at room temperature and under vacuum, stir for one hour, and then blow in argon And add 400 milligrams of rhodamine 6G, 600 microliters of triethylamine and 150 milligrams of cuprous iodide successively, this reaction solution is diluted with 10 milliliters of ethyl acetates after stirring at room temperature for 24 hours, then successively with 20 milliliters of deionized water , 5 milliliters of concentration is the sodium hydroxide solution washing of 1 mol / liter; b. the mixture is separated with separatory funnel, and the organic phase obtained is dried with anhydrous magnesium sulfate first, and dichloromethane:tetrahydrofuran=2:1 volume Prepare an eluent, then pass the dried crude fluorescent probe through the column with the eluent, remove the solvent by rotary evaporation at 35°C, and dry in vacuum to obtain a purple-red product.
Embodiment 2
[0065] a. Dissolve 200 milligrams of potassium selenide in 10 milliliters of N, N-dimethylformamide, then add 200 milligrams of o-trifluoromethylbromobenzene at room temperature and under vacuum, stir for one hour, feed nitrogen and Add 500 milligrams of rhodamine 6G, 500 microliters of triethylamine and 190 milligrams of cuprous iodide successively, the reaction solution was stirred at room temperature for 24 hours and then diluted with 30 milliliters of ethyl acetate, and then respectively washed with 5 milliliters of deionized water, Wash with 20 ml of sodium hydroxide solution with a concentration of 1 mol / L; b. separate the mixture with a separatory funnel, and dry the obtained organic phase with anhydrous magnesium sulfate to obtain dichloromethane:tetrahydrofuran=5:1 volume ratio Prepare an eluent, then pass the dried fluorescent probe crude product through the column with the eluent, remove the solvent by rotary evaporation at 45°C, and distill to obtain a purple-red pr...
Embodiment 3
[0067] a. Dissolve 170 mg of potassium selenide in 10 ml of N,N-dimethylformamide, then add 220 mg of o-trifluoromethyl bromide under vacuum at room temperature, stir for one hour, and then inject helium And add 450 milligrams of rhodamine 6G, 550 microliters of triethylamine and 170 milligrams of cuprous iodide successively, this reaction solution is diluted with 20 milliliters of ethyl acetates after stirring at room temperature for 24 hours, then successively with 10 milliliters of deionized water , 10 milliliters concentration is the sodium hydroxide solution washing of 1 mol / liter; b. the mixture is separated with separatory funnel, the organic phase that obtains is dried with anhydrous magnesium sulfate earlier, take methanol: ethyl acetate=1:20 volume Prepare the eluent, then pass the dried crude fluorescent probe through the column with the eluent, remove the solvent by rotary evaporation at 40°C, and dry in vacuo to obtain a purple-red product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com