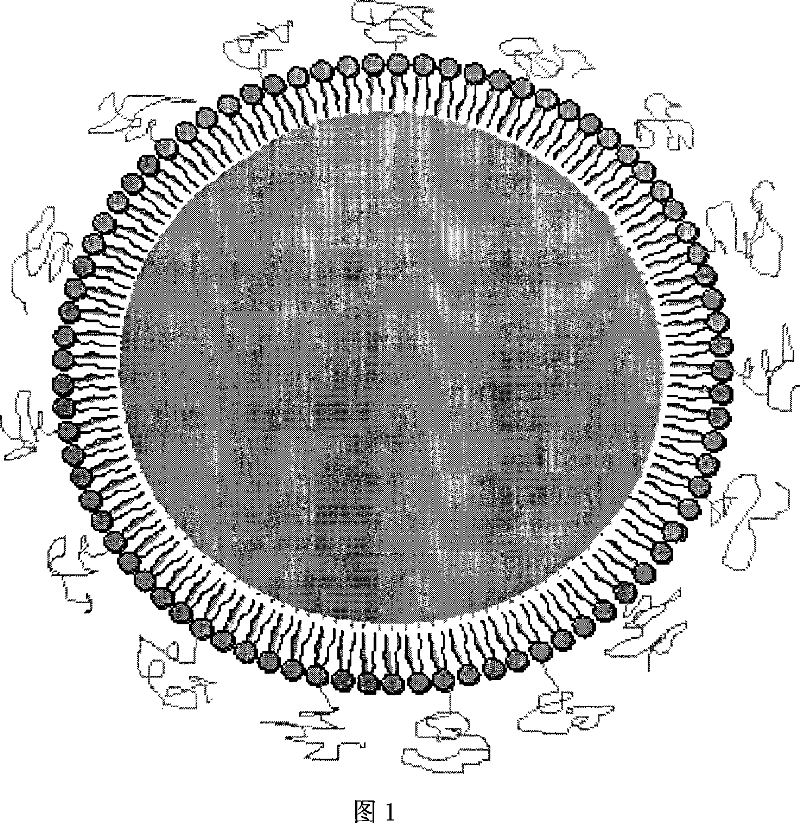
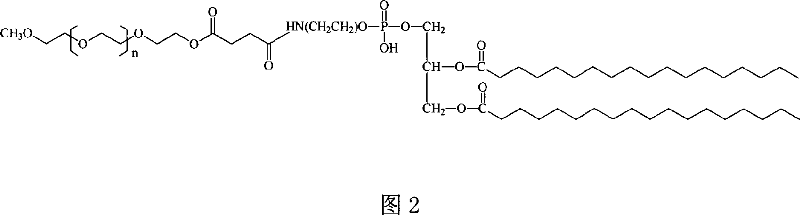
Superregulated long-cycled lipid emulsion carrying medicine reagent for mainline
A technology of fat emulsion and long circulation, which is applied in the direction of medical preparations with non-active ingredients, oil/fat/wax non-active ingredients, medical preparations containing active ingredients, etc., which can solve the problem of increasing the passive targeting function of drugs and poor stability and other issues to achieve the effect of enhancing bioavailability and clinical therapeutic effect and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0044] Example 1: Long Circulating Lipid Emulsion Pharmaceutical Formulation with Minimal PEG-phospholipid Content
[0045] soybean oil (for injection)
[0046] Take 100.0 g of soybean oil, add 12.0 g of lecithin, 0.4 g of sodium oleate, and 0.2 g of polyethylene glycol-phospholipid (PEG-DSPE), and heat to 75°C under nitrogen protection, and stir for about 10 minutes to make the added Various lipid compounds are fully dissolved, then add 10ml vitamin E, dissolve and mix well. Another 800ml of water for injection was taken, and 22g of glycerol was added. Under the condition of nitrogen protection, the oil solution containing the drug was added into the glycerol aqueous solution under the condition of shearing and stirring to prepare colostrum, and the total amount was adjusted to 1000ml. The high-pressure homogenizer homogenizes 5-8 times, the homogenization pressure is about 100MPa, and the particle size ranges from 150nm to 300nm, adjust the pH to 7.0-8.0, filter, ...
example 2
[0047] Example 2: Long Circulating Lipid Emulsion Pharmaceutical Formulation with Highest PEG-Phospholipid Content
[0048] soybean oil (for injection)
100.0g
Lecithin (for injection)
10.0g
sodium oleate
0.4g
polyethylene glycol-phospholipid
8.0g
Vitamin E (for injection)
10ml
Glycerin
22g
Water for Injection
Add to 1000ml
[0049] Take 100.0 g of soybean oil, add 10.0 g of lecithin, 0.4 g of sodium oleate, and 8.0 g of polyethylene glycol-phospholipid (PEG-DSPE), heat to 75°C under nitrogen protection, and stir for about 10 minutes to make the added Various lipid compounds are fully dissolved, then add 10ml vitamin E, dissolve and mix well. Another 800ml of water for injection was taken, and 22g of glycerol was added. Under the condition of nitrogen protection, the oil solution containing the drug was added into the glycerol aqueous solution under the condition of shearing and ...
example 3
[0051] Example 3: The long-circulation fat emulsion drug preparation prepared by the minimum content of animal and vegetable oil, liquid oily drug or oil solution as drug solvent (only using soybean oil as an example)
[0052] soybean oil (for injection)
[0053] Take 50.0g of soybean oil, add 10.0g of lecithin, 0.4g of sodium oleate, 4.0g of polyethylene glycol-phospholipid (PEG-DSPE), and heat to 75°C under nitrogen protection, and stir for about 10min to make the added Various lipid compounds are fully dissolved, then add 10ml vitamin E, dissolve and mix well. Another 800ml of water for injection was taken, and 22g of glycerol was added. Under the condition of nitrogen protection, the oil solution containing the drug was added into the glycerol aqueous solution under the condition of shearing and stirring to prepare colostrum, and the total amount was adjusted to 1000ml. The high-pressure homogenizer homogenizes 5-8 times, the homogenization pressure is about 100...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com