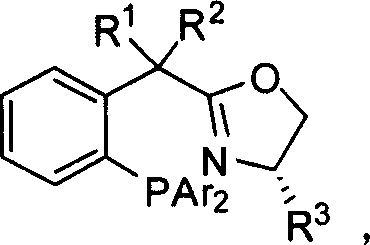
Benzyl-position-substituted oxazoline phosphine ligand with chirality center, synthetic method and application
A technology of oxazoline and phosphine ligands, which is applied in the field of benzyl substituted oxazoline phosphine ligands, and can solve problems such as unsatisfactory results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Synthesis of Ligand P1
[0030] In a 100mL three-necked flask, 375mg (5mmol) of L-alaninol was dissolved in 40mL of THF, and n-BuLi (1.6M, 6.3mL, 10mmol) was added dropwise at 0°C. Lithiation was carried out at room temperature (22°C) for 1 hour after the addition was completed. 1.670g (5mmol) of 2-o-diphenylphosphobenzyl-4-isopropyl-4,5-dihydrooxazole was dissolved in 10mL THF, added dropwise to the above solution, heated to reflux for 27 hours, and obtained by column chromatography Corresponding amide compound; This amide compound is dissolved in 20mLCH 2 Cl 2, add 2.3mL (16.1mmol) of triethylamine, 0.25mL (3.22mmol) of trifluoromethanesulfonyl chloride, stir at room temperature for 24 hours, and separate by column chromatography, P1 is viscous liquid 895mg (72%);
[0031] P1(R 1 = R 2 = H, R 3 =CH 3 , Ar=Ph):
[0032] 1 H NMR (300MHz, CDCl 3 ): δ1.21(d, J=6.6Hz, 3H), 3.69-3.82(m, 2H), 3.88-4.05(m, 3H), 6.88-6.92(m, 1H), 7.14-7.38(m, 13H ); 31 P ...
Embodiment 2
[0039] Example 2: Synthesis of Ligand P5
[0040]
[0041] Under the protection of argon, (S)-2-(2-(2-bromophenyl)2-propyl)-4-isopropyl-oxazoline 997mg (3.24mmol) was dissolved in 15mLTHF, dry ice-acetone Cool the bath to -78°C, add n-BuLi2.31mL (3.70mmol) dropwise, lithiate at low temperature for 1 hour, add PPh dropwise at this temperature 2 Cl0.67mL (3.70mmol), after maintaining the low temperature for 30 minutes, remove the cold bath, rise to room temperature and react for 1 hour, quench the reaction with 15mL of saturated ammonium chloride, separate the organic layer, extract the aqueous layer with ether, and dry over anhydrous sodium sulfate , filtered, removed the solvent, and separated by column chromatography to obtain 876 mg of P5 as a white solid (66%).
[0042] P5(R 1 = R 2 =CH 3 ,R 3 =CH(CH 3 ) 2 , Ar=Ph):
[0043] 1 H NMR (300MHz, CDCl 3 ): δ0.76(d, J=7.0Hz, 3H), 0.84(d, J=7.0Hz, 3H), 1.73(m, 1H), 1.77(s, 3H), 1.84(s, 3H), 3.60 -3.70(m, 3H), 7.15-7....
Embodiment 3
[0058] Example 3: Synthesis of Ligands in Structure 2
[0059] With ligand P13 (n=0,, R 3 =CH(CH 3 ) 2 , the synthesis of Ar=Ph) is an example, completed by the following five-step reaction:
[0060]
[0061] 1.985g (10.1mmol) of o-bromophenylacetonitrile, 1.73mL (20.1mmol) of 1,2-dibromoethane were mixed and dissolved in 20mL MeCN, and 3.294g (10.2mmol) of n-tetrabutylammonium bromide was added under ice-water bath conditions, 50 % NaOH aqueous solution 20mL, stirred at room temperature for 1.5 hours, added 20mL of water, separated the organic layer, extracted the aqueous layer with ethyl acetate, dried over anhydrous sodium sulfate, filtered, spin-dried, and obtained 1-o-bromophenyl-1- Nitrile-cyclopropane white solid 1.840 g (82%);
[0062] 1 H NMR (300MHz, CDCl 3 ): δ1.25-1.39(m, 2H), 1.66-1.81(m, 2H), 7.16-7.34(m, 3H), 7.57-7.60(m, 1H); 13 C NMR (300MHz, CDCl 3 ): δ15.2, 16.6, 121.5, 126.3, 127.6, 130.1, 131.4, 133.1, 134.9; EI-MS m / z (relative intensity): 221 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Relative strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com