Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
30 results about "Treatments for overactive bladder" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Treatments for overactive bladder are therapies used to treat overactive bladder or related conditions, such as urinary incontinence and frequent urination. Drugs are commonly prescribed for this. Other non-drug treatments are also used.
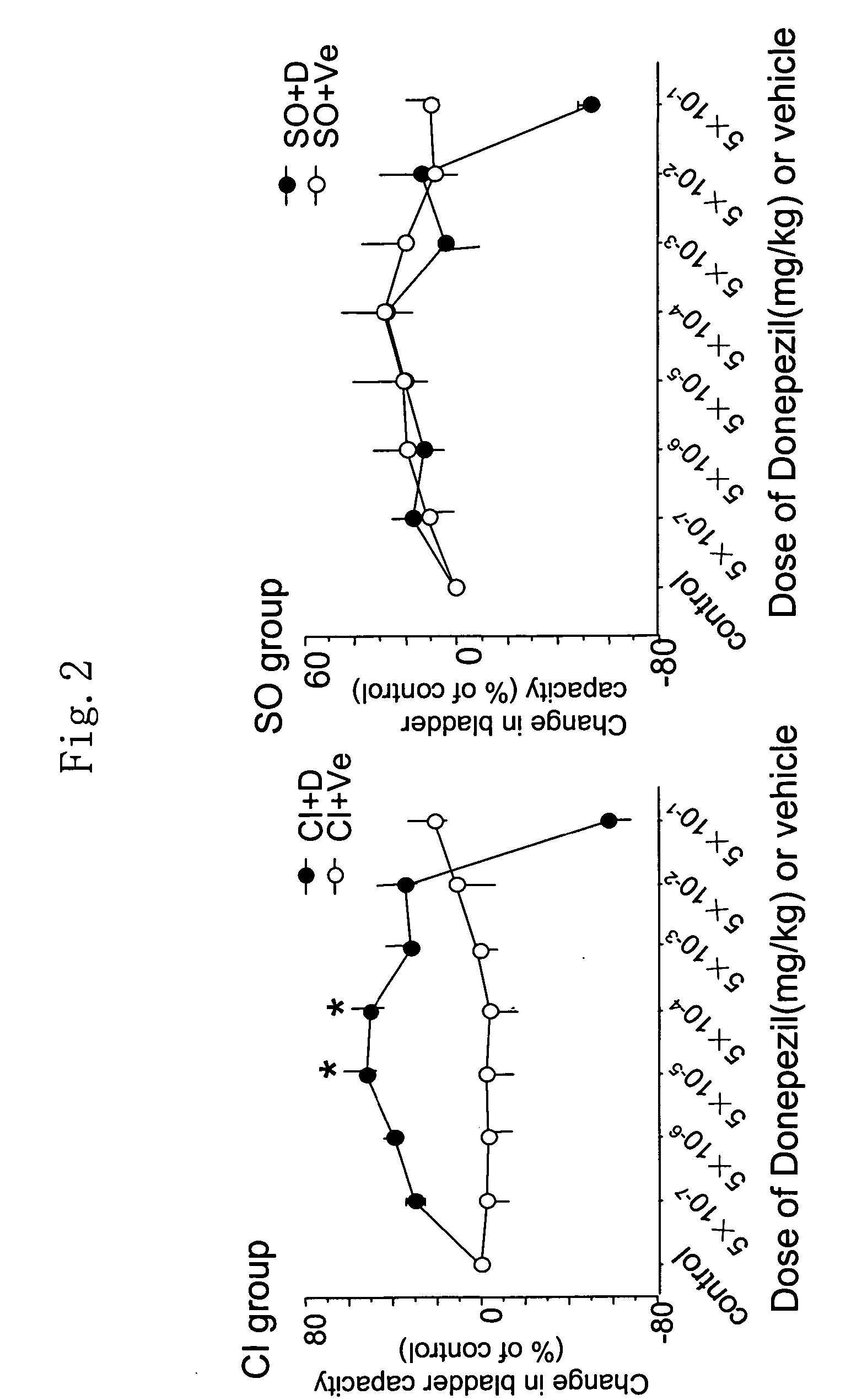
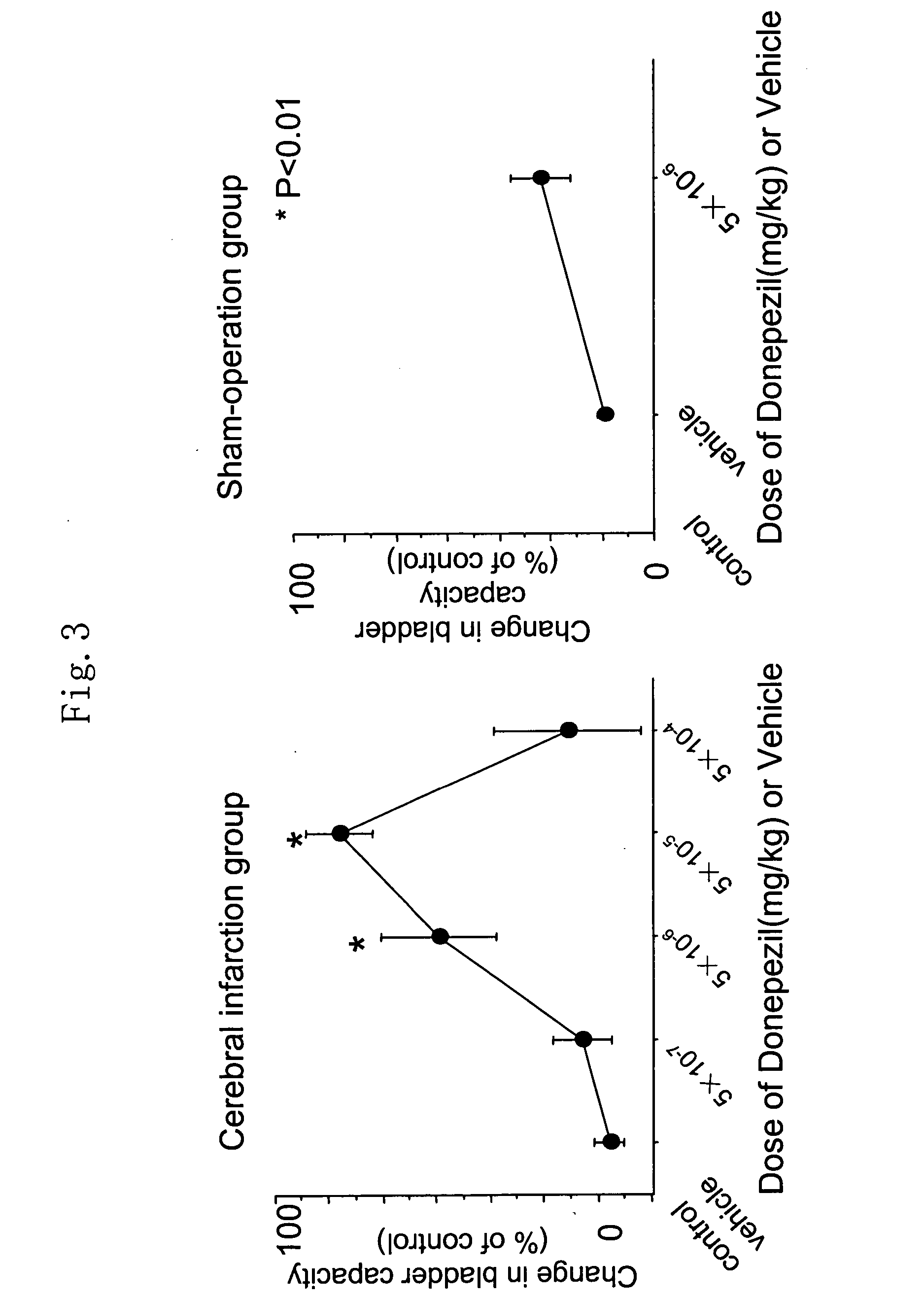
Therapeutic agent for overactive bladder resulting from cerebral infarction
An agent for treating overactive bladder resulting from cerebral infarction, comprising administrating a compound having a cholinesterase inhibitory activity or a pharmacologically acceptable salt thereof.
Owner:EISIA R&D MANAGEMENT CO LTD
Pharmaceutical composition for treating overactive bladder
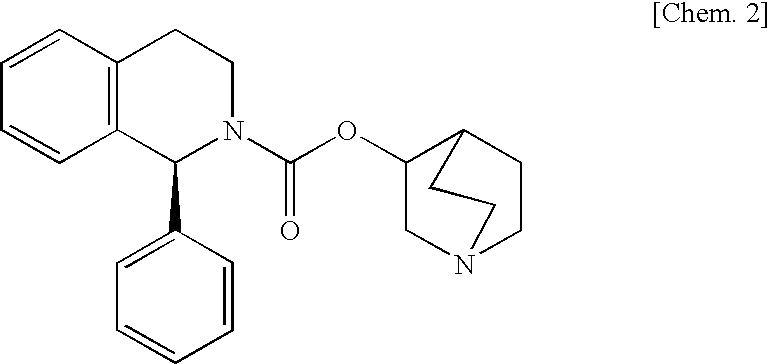
[Problems] To provide a pharmaceutical composition which is useful as a therapeutic agent for overactive bladder.[Means for Solution] A pharmaceutical composition comprising (R)-2-(2-aminothiazol-4-yl)-4′-{2-[(2-hydroxy-2-phenylethyl)amino]ethyl}acetanilide or a pharmaceutically acceptable salt thereof and (3R)-quinuclidin-3-yl (1S)-1-phenyl-1,2,3,4-tetrahydroisoquinoline-2-carboxylate or a pharmaceutically acceptable salt thereof, as active ingredients, in particular a pharmaceutical composition for improving various symptoms accompanying overactive bladder, such as urinary urgency, pollakiuria and / or urinary incontinence
Owner:ASTELLAS PHARMA INC
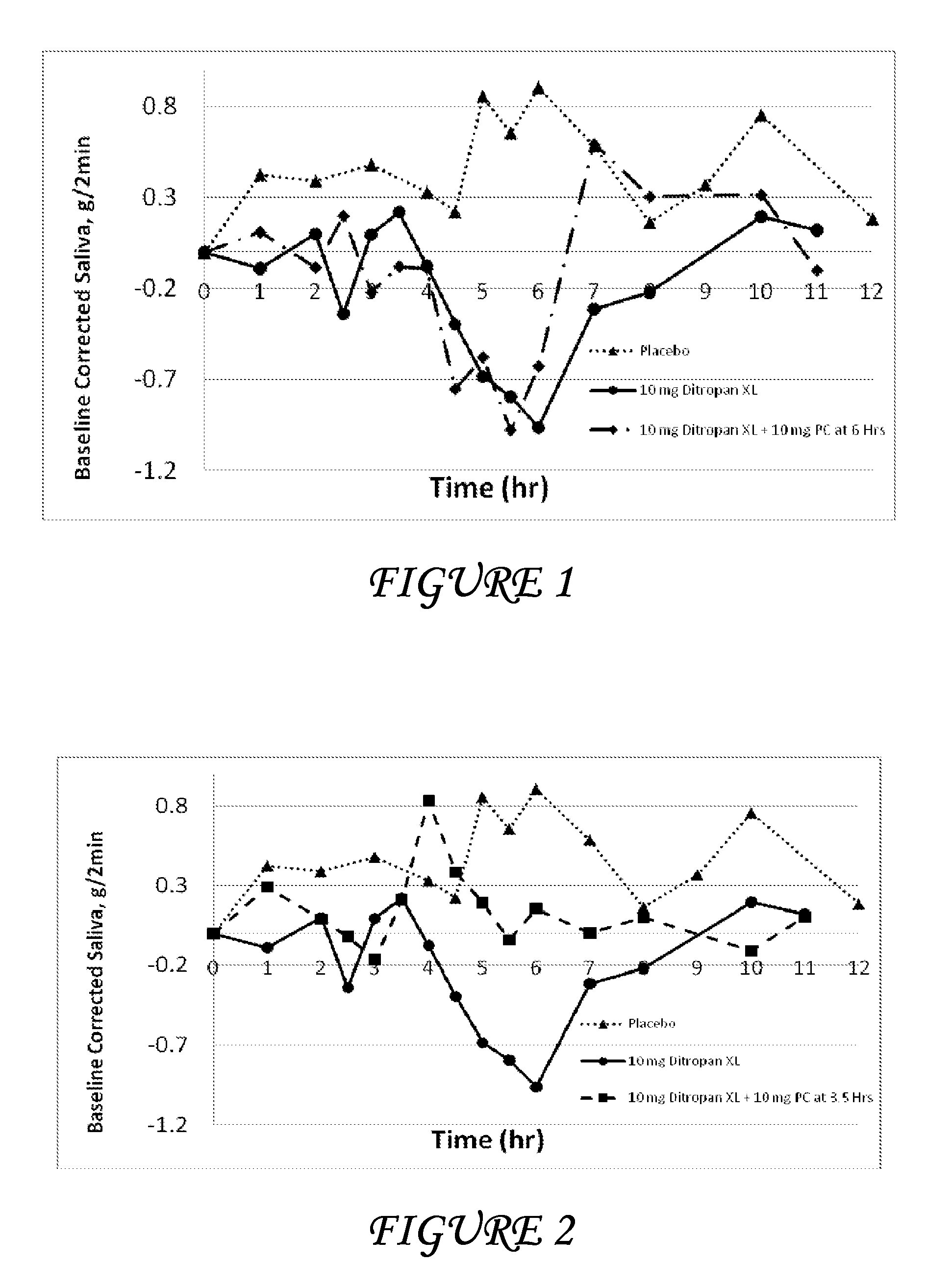
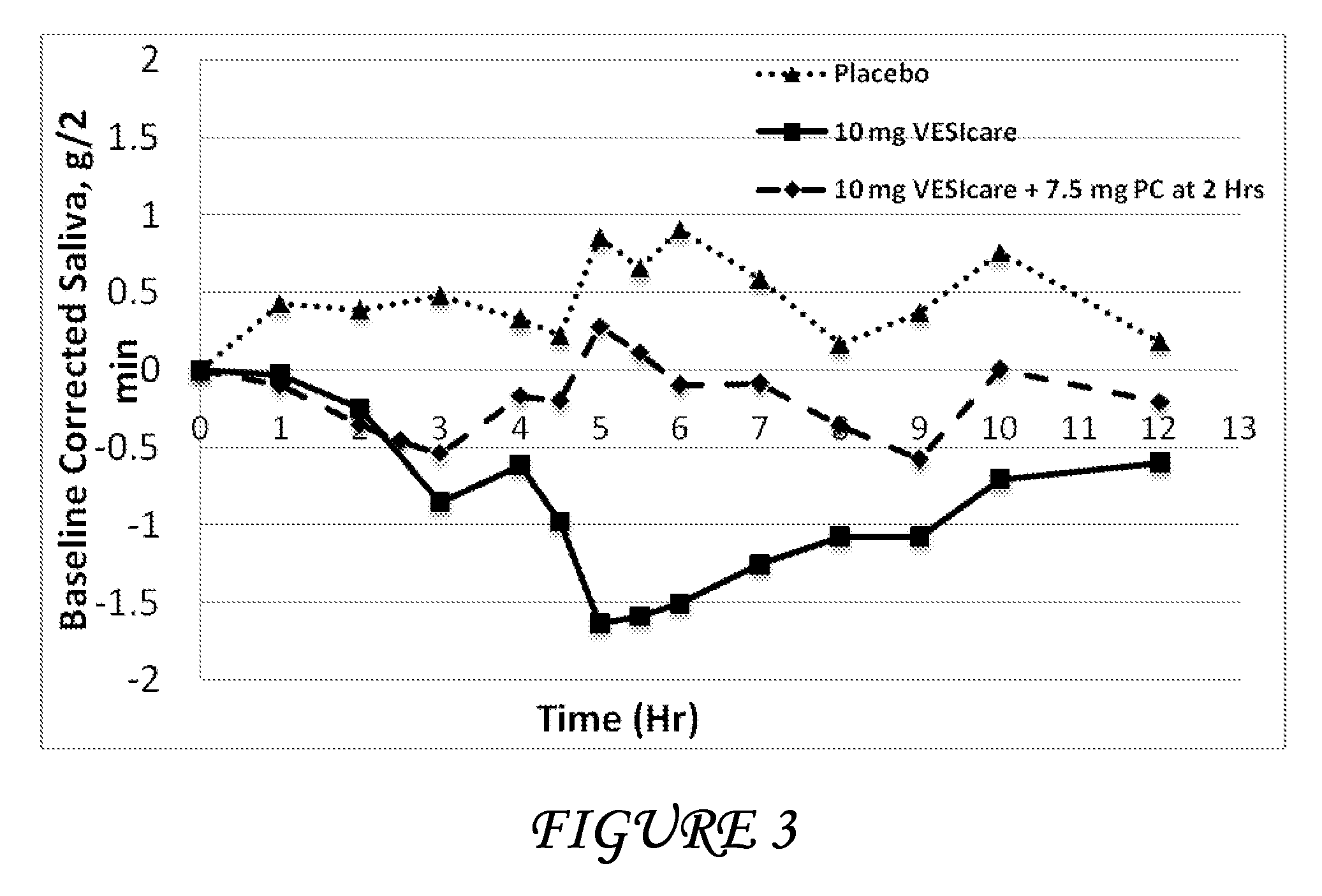
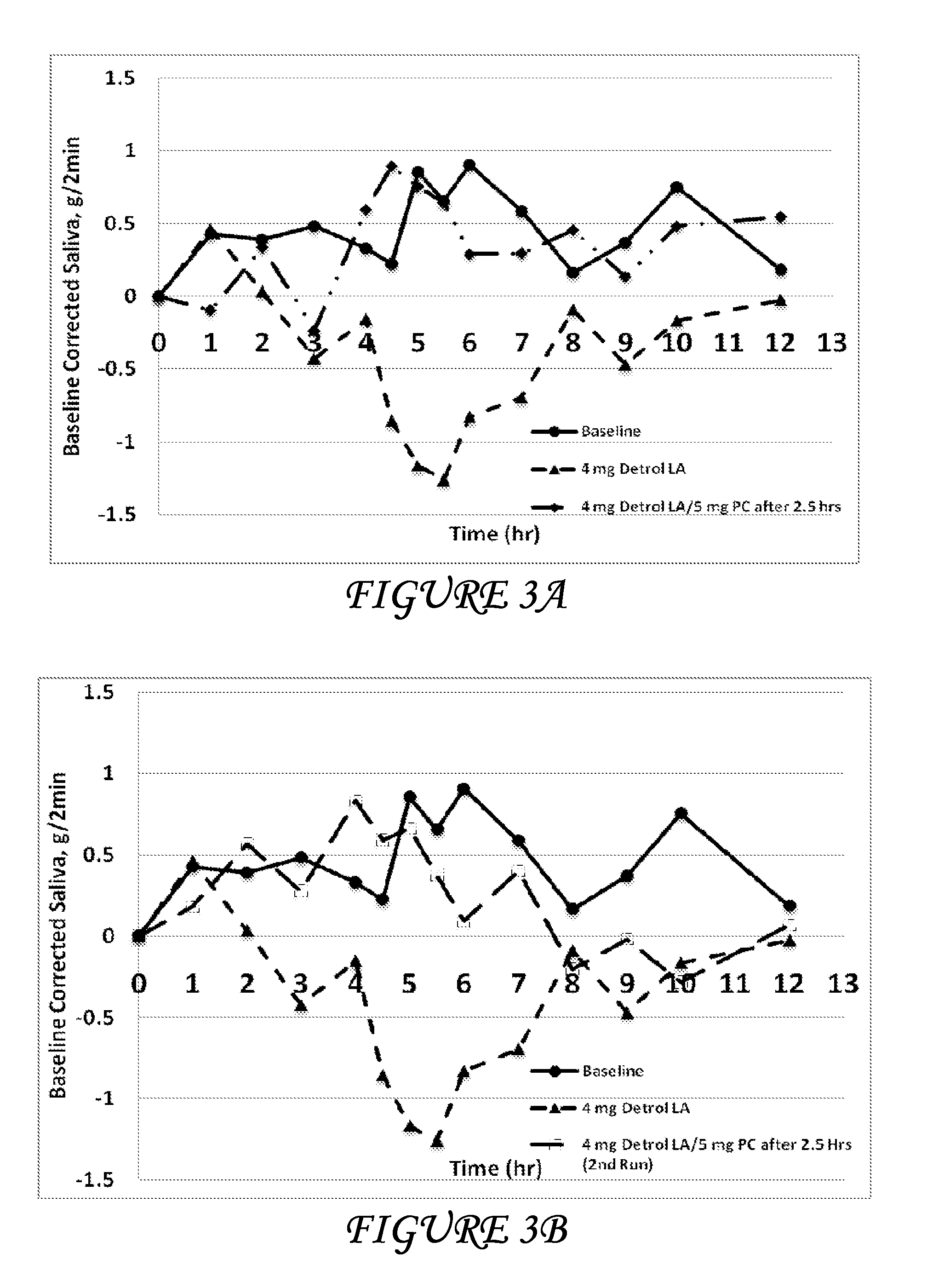
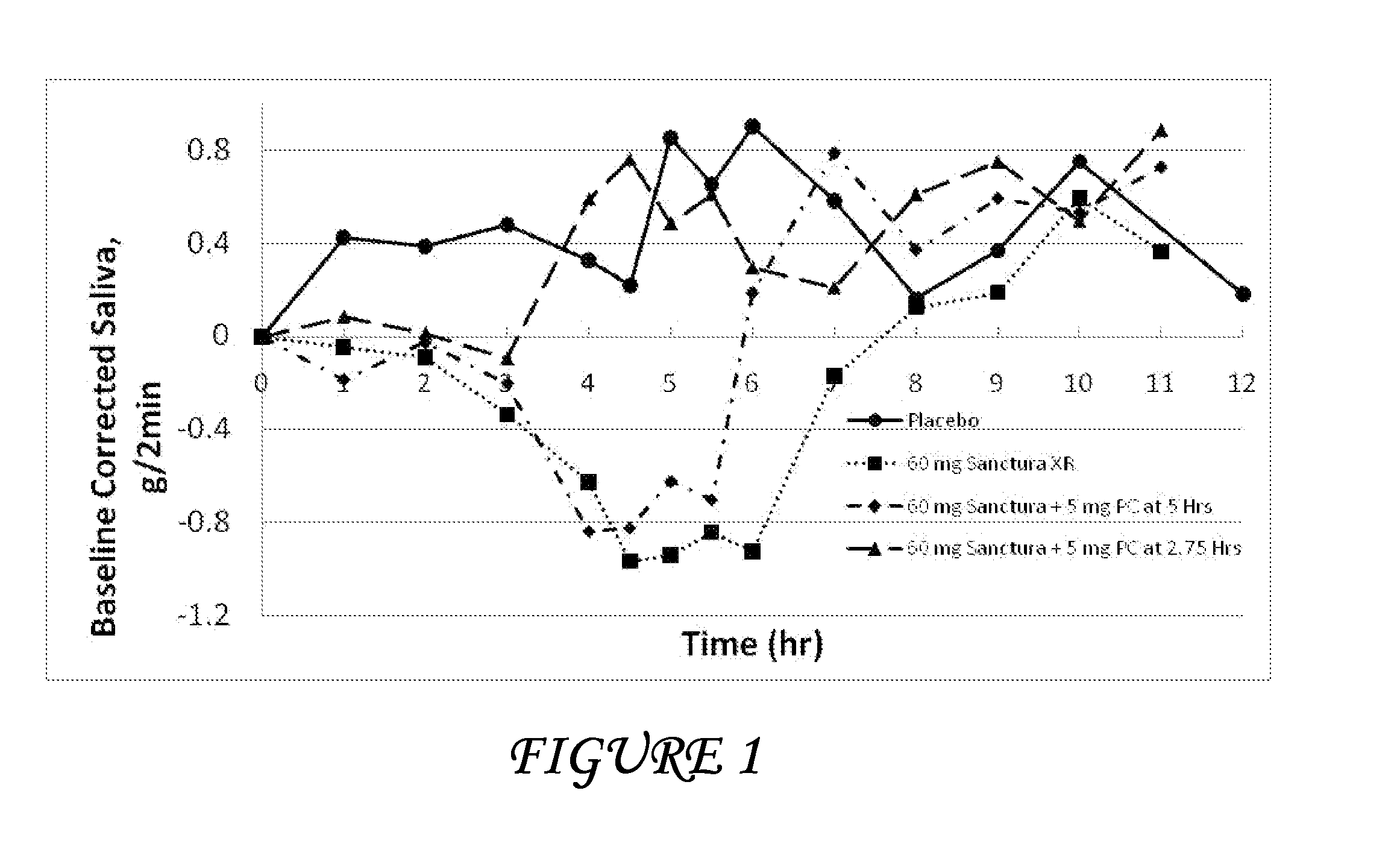
Combinations of oxybutynin and salivary stimulants for the treatment of overactive bladder
Disclosed herein are pharmaceutical compositions comprising a therapeutically effective amount of extended release oxybutynin, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof. Also disclosed herein are methods of treating a patient suffering from overactive bladder, the method comprising identifying a patient in need thereof, and administering to the patient a therapeutically effective amount of extended release oxybutynin, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof. Also disclosed herein are methods of alleviating a side effect of treatment for overactive bladder in a patient suffering therefrom, the method comprising identifying a patient in need thereof, and administering to the patient a therapeutically effective amount of extended release oxybutynin, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof.
Owner:THERAVIDA INC
Remedy for overactive bladder comprising acetic acid anilide derivative as the active ingredient
InactiveUS20090093529A1Strong bladder relaxation actionDecreases contraction frequency of contractionBiocideOrganic active ingredientsMeasurement testBULK ACTIVE INGREDIENT
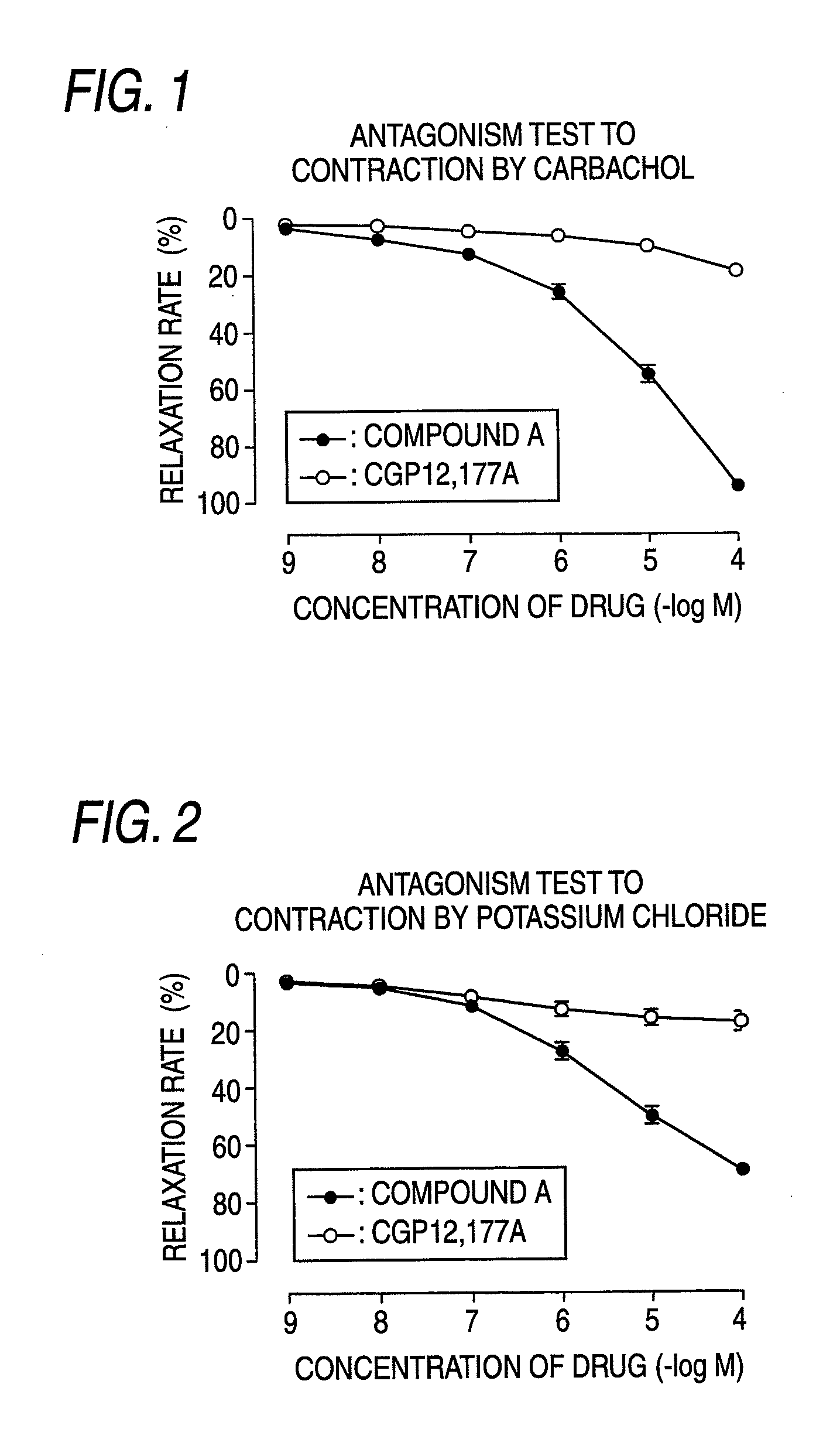
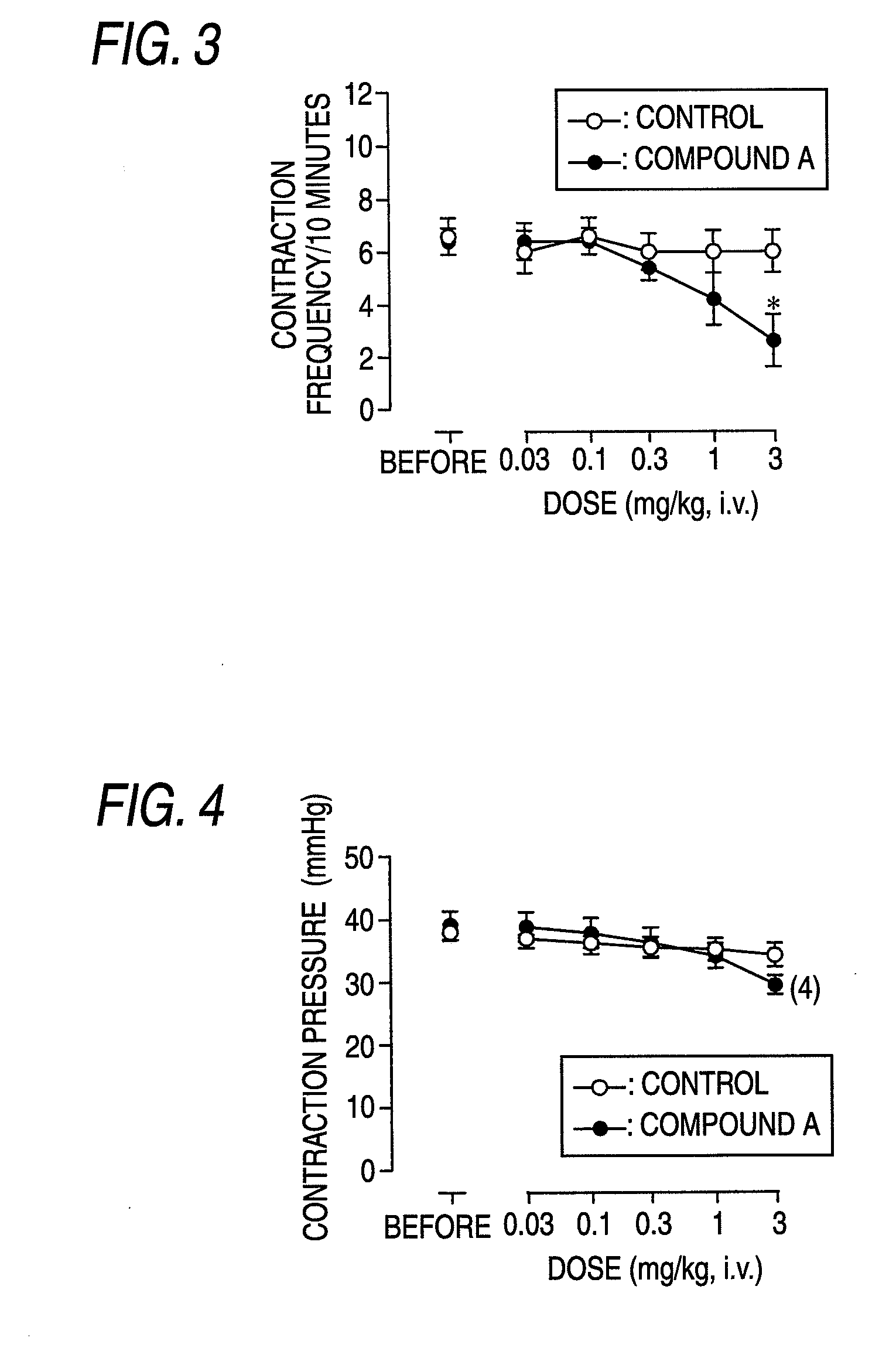
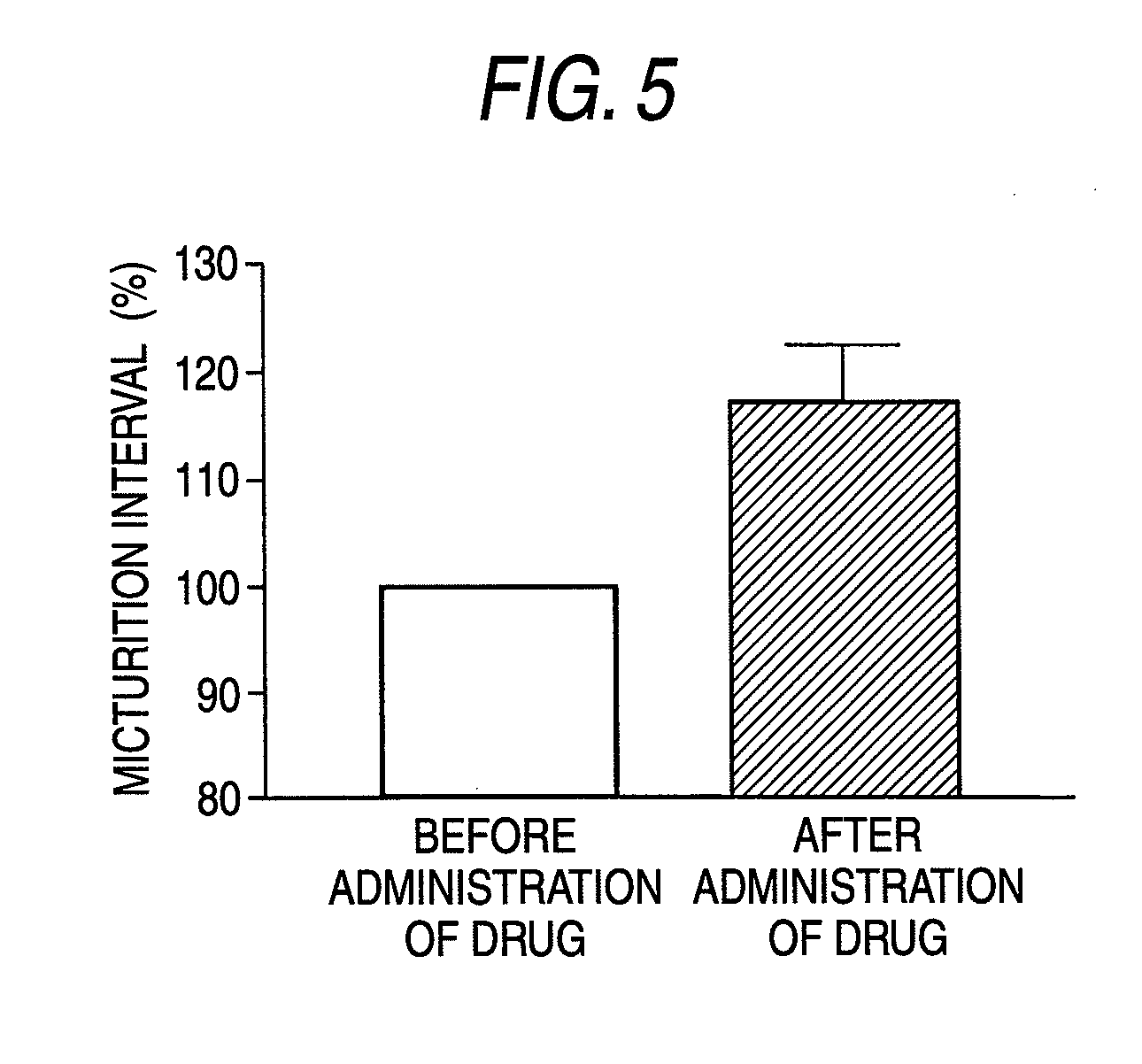
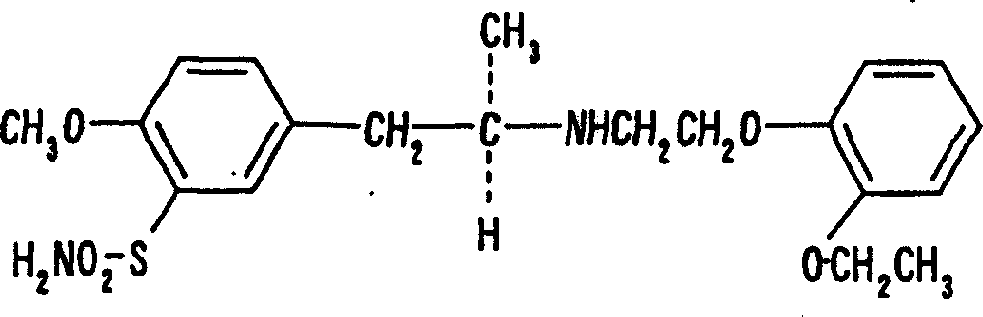
(R)-2-(2-aminothiazol-4-yl)-4′-[2-[(2-hydroxy-2-phenylethyl)amino]ethyl]acetic acid anilide or its salt shows a potent bladder relaxation effect in “isolated rat bladder smooth muscle relaxation test”, dose-dependently lowers the contraction frequency of rhythmic bladder contractions in “rat rhythmic bladder contraction measurement test” and, moreover, prolongs the urination intervals in “urination functions measurement test on cyclophosphamide-induced overactive bladder model rat”. Owing to these effects, the above compound is useful as a remedy for overactive bladder.
Owner:ASTELLAS PHARMA INC
Pharmaceutical compositions and the treatment of overactive bladder
ActiveUS20170151199A1Decrease micturition frequencyOrganic active ingredientsAdrenergicTreatments for overactive bladder
The present invention relates to methods of treating overactive bladder and the symptoms associated therewith, for example, urinary urgency, frequency of mictruitions, nocturia, and urgency urinary incontinence. One treatment method according to the present invention comprises treatment with the beta-3 adrenergic receptor agonist solabegron. Another treatment combination according to the invention comprises solabegron, and a muscarinic receptor antagonist which results in a synergistic effect on the symptoms associated with OAB.
Owner:B3AR THERAPEUTICS INC
Therapeutic agent for overactive bladder
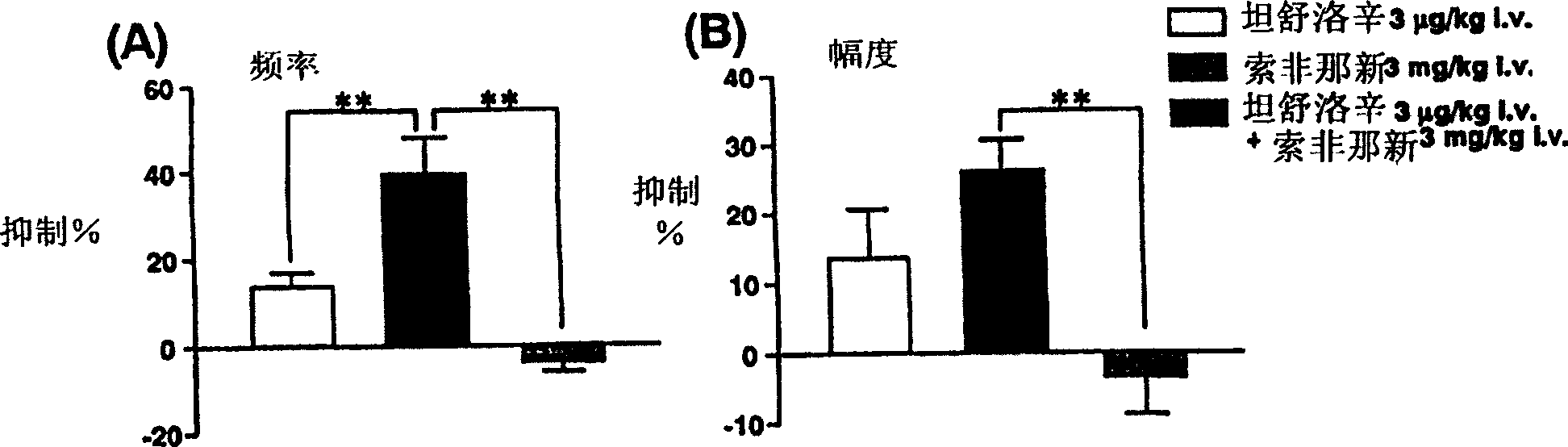
A medicinal composition for treatments for overactive bladder which contains tamsulosin or a pharmaceutically acceptable salt thereof as an active ingredient.
Owner:ASTELLAS PHARMA INC
Combinations of solifenacin and salivary stimulants for the treatment of overactive bladder
Disclosed herein are pharmaceutical compositions comprising a therapeutically effective amount of extended release solifenacin, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof. Also disclosed herein are methods of treating a patient suffering from overactive bladder, the method comprising identifying a patient in need thereof, and administering to the patient a therapeutically effective amount of extended release solifenacin, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof. Also disclosed herein are methods of alleviating a side effect of treatment for overactive bladder in a patient suffering therefrom, the method comprising identifying a patient in need thereof, and administering to the patient a therapeutically effective amount of extended release solifenacin, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof.
Owner:THERAVIDA INC
Pharmaceutical compositions and the treatment of overactive bladder
The present invention relates to methods of treating overactive bladder and the symptoms associated therewith, for example, urinary urgency, frequency of mictruitions, nocturia, and urgency urinary incontinence. One treatment method according to the present invention comprises treatment with the beta-3 adrenergic receptor agonist solabegron. Another treatment combination according to the invention comprises solabegron, and a muscarinic receptor antagonist which results in a synergistic effect on the symptoms associated with OAB.
Owner:B3AR THERAPEUTICS INC
Combinations of solifenacin and salivary stimulants for the treatment of overactive bladder
Disclosed herein are pharmaceutical compositions comprising a therapeutically effective amount of extended release solifenacin, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof. Also disclosed herein are methods of treating a patient suffering from overactive bladder, the method comprising identifying a patient in need thereof, and administering to the patient a therapeutically effective amount of extended release solifenacin, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof. Also disclosed herein are methods of alleviating a side effect of treatment for overactive bladder in a patient suffering therefrom, the method comprising identifying a patient in need thereof, and administering to the patient a therapeutically effective amount of extended release solifenacin, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof.
Owner:THERAVIDA INC
Combinations of solifenacin and salivary stimulants for the treatment of overactive bladder
Disclosed herein are pharmaceutical compositions comprising a therapeutically effective amount of extended release solifenacin, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof. Also disclosed herein are methods of treating a patient suffering from overactive bladder, the method comprising identifying a patient in need thereof, and administering to the patient a therapeutically effective amount of extended release solifenacin, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof. Also disclosed herein are methods of alleviating a side effect of treatment for overactive bladder in a patient suffering therefrom, the method comprising identifying a patient in need thereof, and administering to the patient a therapeutically effective amount of extended release solifenacin, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof.
Owner:THERAVIDA INC
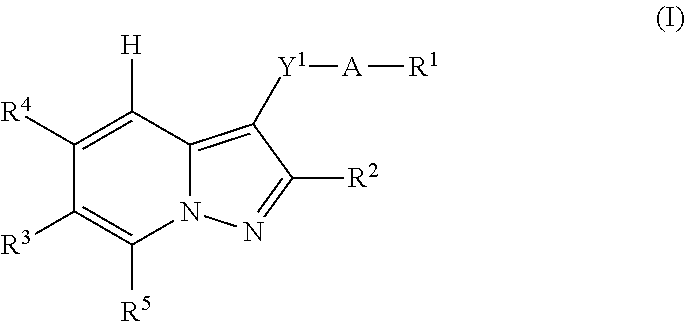
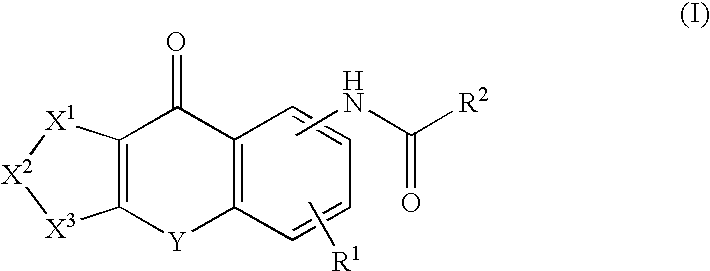
Pyrazolopyridine derivative or pharmacologically acceptable salt thereof
InactiveUS20130331378A1Strong EP receptor antagonistic effectLow toxicityBiocideOrganic chemistryAntagonismReceptor
A pyrazolopyridine derivative represented by the following formula (I) or a pharmacologically acceptable salt thereof exhibits a strong EP1 receptor antagonistic effect. Thus, the derivative or the pharmacologically acceptable salt is useful as a therapeutic agent for lower urinary tract symptoms (LUTS), particularly, overactive bladder syndrome (OABs), or a prophylactic agent therefor and furthermore, is also useful in the treatment, prevention, or suppression of various pathological conditions in which the EP1 receptor is involved, such as inflammatory disease, pain disease, osteoporosis, and cancer.[A is a benzene ring or the like, Y1 is C1-6 alkylene, R1 is —C(═O)—OZ1 or the like, Z1 is H or the like, R2 is a branched C3-6 alkyl group or the like, R3 is H or the like, R4 is a hydrogen atom or the like, and R5 is a hydrogen atom or the like].
Owner:KYORIN PHARMA CO LTD +1
Combinations of tolterodine and salivary stimulants for the treatment of overactive bladder
Disclosed herein are pharmaceutical compositions comprising a therapeutically effective amount of extended release tolterodine, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof. Also disclosed herein are methods of treating a patient suffering from overactive bladder, the method comprising identifying a patient in need thereof, and administering to the patient a therapeutically effective amount of extended release tolterodine, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof. Also disclosed herein are methods of alleviating a side effect of treatment for overactive bladder in a patient suffering therefrom, the method comprising identifying a patient in need thereof, and administering to the patient a therapeutically effective amount of extended release tolterodine, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof.
Owner:THERAVIDA INC
Catheter for radio frequency treatment for overactive bladder and matched device thereof
PendingCN112244997APrecise positioningAddresses the need for long-term treatment leading to poor adherenceBalloon catheterMulti-lumen catheterHyperactivity bladderEngineering
The present invention discloses a catheter for radio frequency treatment for overactive bladder and a matched device thereof. The catheter for radio frequency treatment for overactive bladder comprises a catheter body and a movable positioning rod, a magnetic block is fixedly arranged at a position, close to a right end, of an inner cavity of the movable positioning rod, and an air bag is arrangedat a position, close to a right side, of an outer surface of the catheter body. In the catheter for radio frequency treatment for overactive bladder, the movable positioning rod is arranged, the magnetic block and a magnetic ring are matched, medical staff can better control a urinary catheterization treatment head to move in a triangular area of a bladder of a patient in vitro, and problems thata traditional treatment means has a poor treatment effect and cost is high are solved.
Owner:THE FIRST AFFILIATED HOSPITAL OF WENZHOU MEDICAL UNIV
Combinations of trospium and salivary stimulants for the treatment of overactive bladder
Disclosed herein are pharmaceutical compositions comprising a therapeutically effective amount of extended release trospium, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof. Also disclosed herein are methods of treating a patient suffering from overactive bladder, the method comprising identifying a patient in need thereof, and administering to the patient a therapeutically effective amount of extended release trospium, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof. Also disclosed herein are methods of alleviating a side effect of treatment for overactive bladder in a patient suffering therefrom, the method comprising identifying a patient in need thereof, and administering to the patient a therapeutically effective amount of extended release trospium, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof.
Owner:THERAVIDA INC
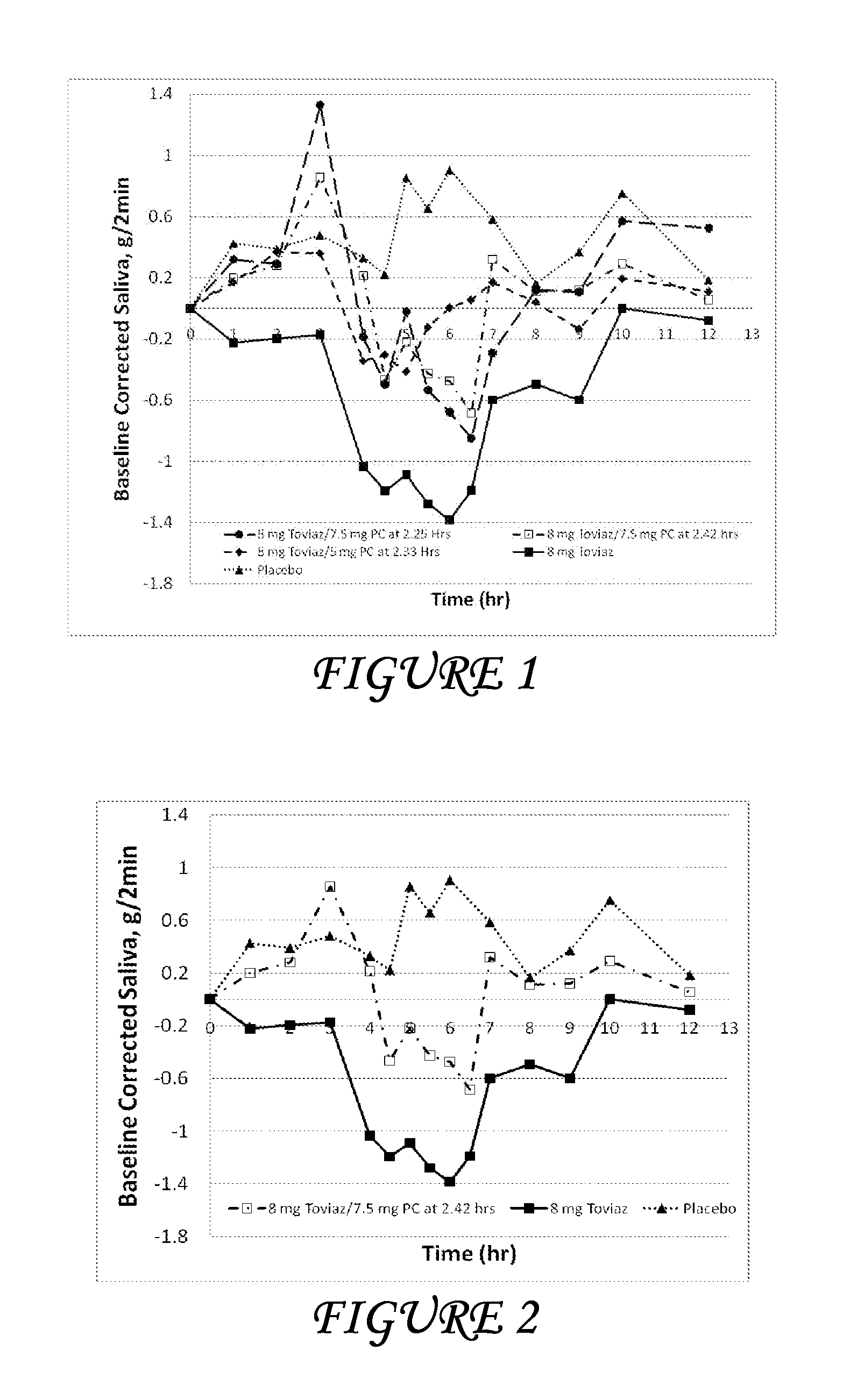
Combinations of fesoterodine and salivary stimulants for the treatment of overactive bladder
Disclosed herein are pharmaceutical compositions comprising a therapeutically effective amount of extended release fesoterodine, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof. Also disclosed herein are methods of treating a patient suffering from overactive bladder, the method comprising identifying a patient in need thereof, and administering to the patient a therapeutically effective amount of extended release fesoterodine, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof. Also disclosed herein are methods of alleviating a side effect of treatment for overactive bladder in a patient suffering therefrom, the method comprising identifying a patient in need thereof, and administering to the patient a therapeutically effective amount of extended release fesoterodine, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof.
Owner:THERAVIDA INC
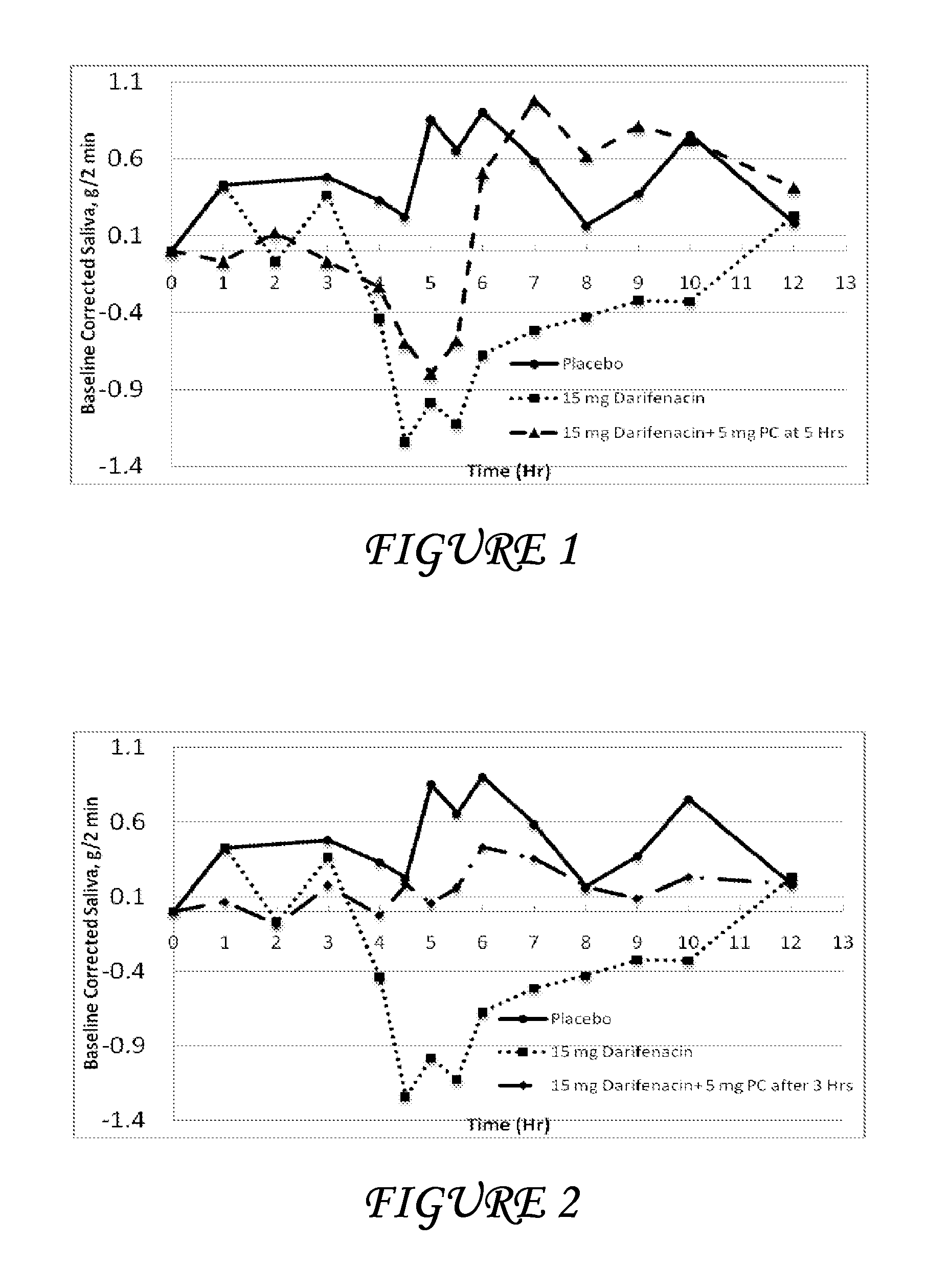
Combinations of darifenacin and salivary stimulants for the treatment of overactive bladder
Owner:THERAVIDA INC
Combinations of oxybutynin and salivary stimulants for the treatment of overactive bladder
Disclosed herein are pharmaceutical compositions comprising a therapeutically effective amount of extended release oxybutynin, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof. Also disclosed herein are methods of treating a patient suffering from overactive bladder, the method comprising identifying a patient in need thereof, and administering to the patient a therapeutically effective amount of extended release oxybutynin, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof. Also disclosed herein are methods of alleviating a side effect of treatment for overactive bladder in a patient suffering therefrom, the method comprising identifying a patient in need thereof, and administering to the patient a therapeutically effective amount of extended release oxybutynin, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof.
Owner:THERAVIDA INC
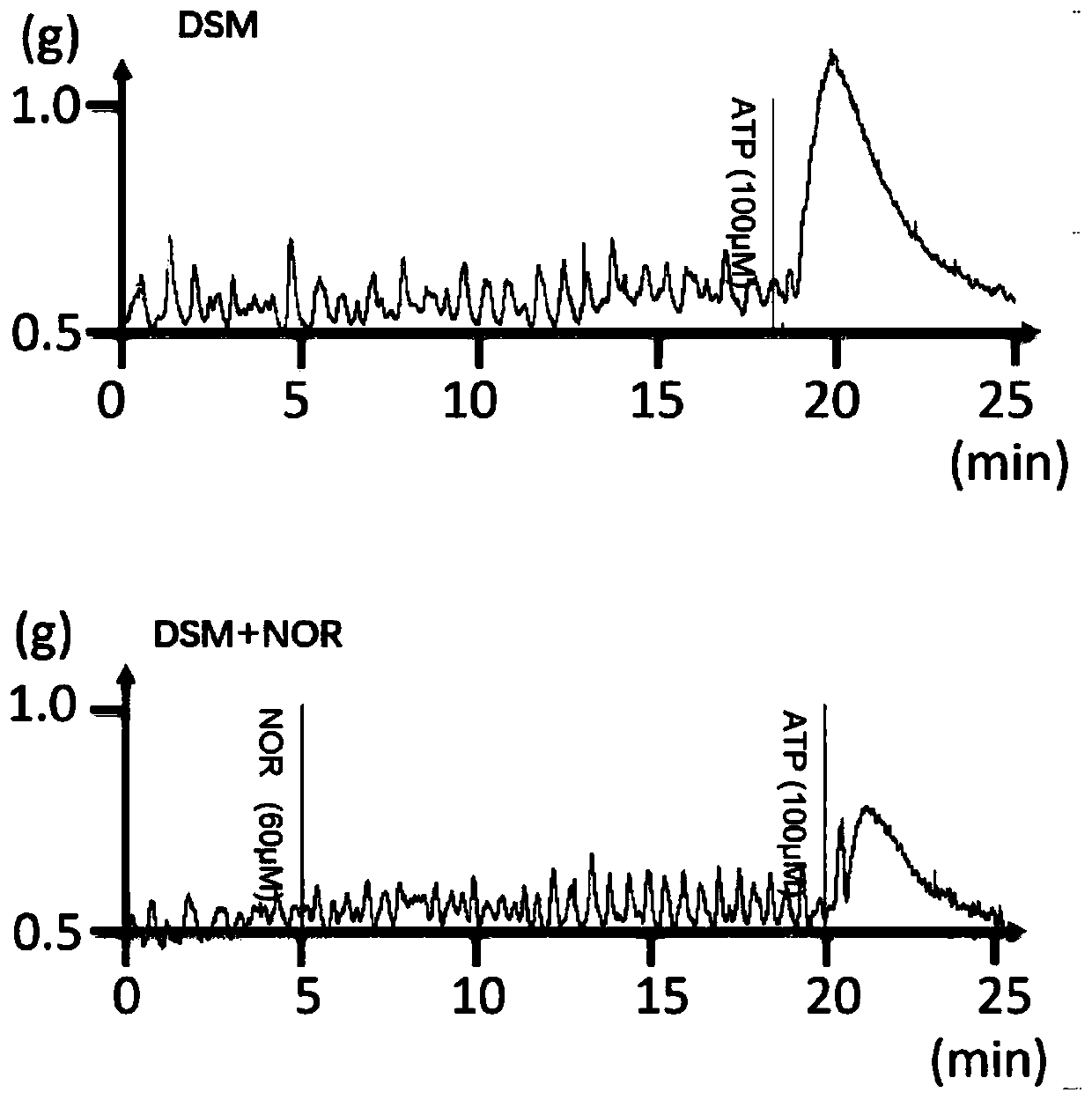
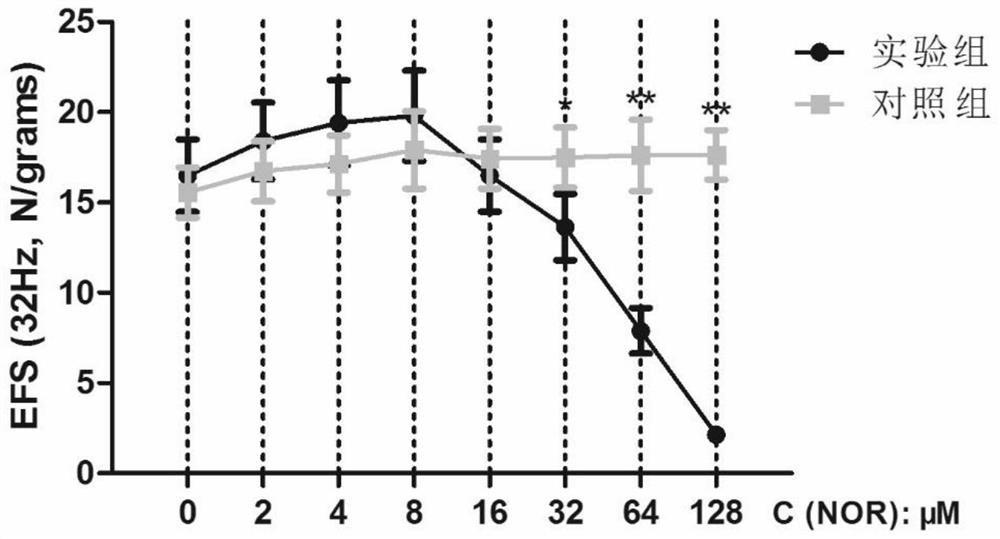
Application of norisoboldine in preparing drugs for preventing and/or treating bladder smooth muscle dysfunction
ActiveCN111184721AEasy to adjustSmall shrinkageOrganic active ingredientsUrinary disorderSmooth muscleAdenosine
The invention provides application of norisoboldine in preparing drugs for preventing and / or treating a bladder smooth muscle dysfunction, and belongs to the technical field of treatment of the bladder smooth muscle dysfunction. The norisoboldine has a very good and obvious regulating function on a bladder smooth muscle function, can lower the contraction ability of detrusor, obviously reduces a contraction response caused to alpha, beta-methylene adenosine 5'-triphosphate (ATP), CCh and KCl by the detrusor, can further obviously reduce contraction caused by electrical field stimulation (EFS),can be used for treating overactive bladder, and can also be used for treating various diseases including uroclepsia, hyperuresis and the like.
Owner:DONGGUAN MATHEMATICAL ENG ACAD OF CHINESE MEDICINE GUANGZHOU UNIV OF CHINESE MEDICINE
Combinations of propiverine and salivary stimulants for the treatment of overactive bladder
InactiveUS20120289547A1Eliminate side effectsBiocidePharmaceutical delivery mechanismSide effectStimulant
Disclosed herein are pharmaceutical compositions comprising a therapeutically effective amount of extended release propiverine, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof. Also disclosed herein are methods of treating a patient suffering from overactive bladder, the method comprising identifying a patient in need thereof, and administering to the patient a therapeutically effective amount of extended release propiverine, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof. Also disclosed herein are methods of alleviating a side effect of treatment for overactive bladder in a patient suffering therefrom, the method comprising identifying a patient in need thereof, and administering to the patient a therapeutically effective amount of extended release propiverine, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof.
Owner:THERAVIDA INC
Traditional Chinese medicine composition for treating overactive bladder as well as preparation process and application
InactiveCN111714598ANo side effectsSignificant effectSkeletal disorderPlant ingredientsCodonopsisRadix Astragali seu Hedysari
The invention discloses a traditional Chinese medicine composition for treating overactive bladder as well as a preparation process and an application. The composition comprises the following components in parts by mass: 40-60 parts of rhizoma dioscoreae, 20-30 parts of radix astragali seu hedysari, 20-30 parts of radix codonopsis, 20-30 parts of fried rhizoma atractylodis macrocephalae, 15-25 parts of poria, 10-20 parts of rhizoma alismatis and 15-25 parts of rhizoma smilacis glabrae. Pharmacodynamic results show that the composition can significantly improve overactive bladder and can be used for treatment of overactive bladder.
Owner:吉林省妇幼保健院
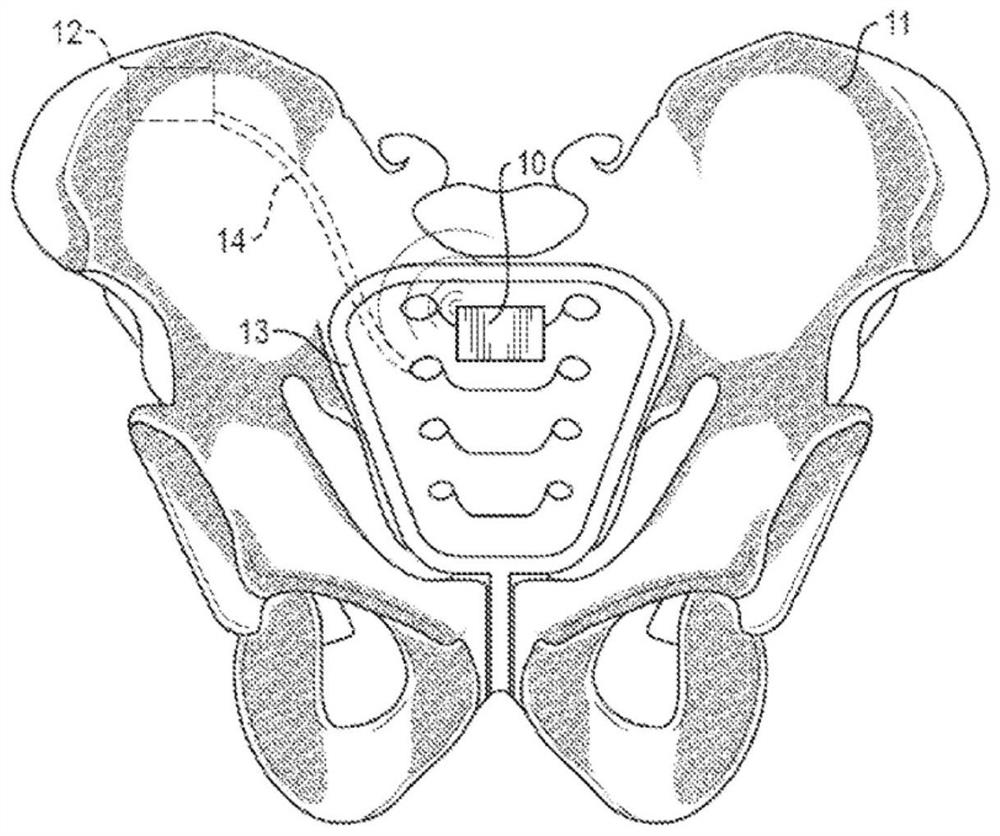
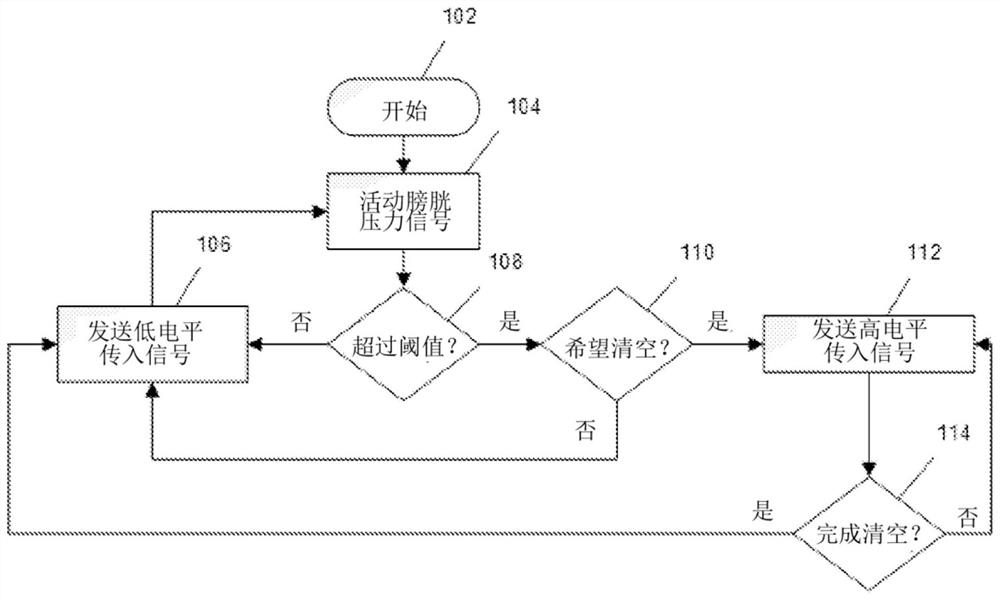
Systems and methods for neurostimulation therapy
ActiveCN107106834BSpinal electrodesExternal electrodesHyperactive bladderTreatments for overactive bladder
The present disclosure relates to methods, devices and systems for neuromodulation. The methods, devices and systems described herein are useful, for example, in the treatment of bladder conditions such as overactive bladder.
Owner:BOSTON SCI SCIMED INC
Application of desmethylisobordine in preparation of medicament for preventing and/or treating bladder smooth muscle dysfunction
ActiveCN111184721BEasy to adjustSmall shrinkageOrganic active ingredientsUrinary disorderSmooth muscleAdenosine
The invention provides application of norisoboldine in preparing drugs for preventing and / or treating a bladder smooth muscle dysfunction, and belongs to the technical field of treatment of the bladder smooth muscle dysfunction. The norisoboldine has a very good and obvious regulating function on a bladder smooth muscle function, can lower the contraction ability of detrusor, obviously reduces a contraction response caused to alpha, beta-methylene adenosine 5'-triphosphate (ATP), CCh and KCl by the detrusor, can further obviously reduce contraction caused by electrical field stimulation (EFS),can be used for treating overactive bladder, and can also be used for treating various diseases including uroclepsia, hyperuresis and the like.
Owner:DONGGUAN MATHEMATICAL ENG ACAD OF CHINESE MEDICINE GUANGZHOU UNIV OF CHINESE MEDICINE
Combinations of trospium and salivary stimulants for the treatment of overactive bladder
Disclosed herein are pharmaceutical compositions comprising a therapeutically effective amount of immediate release trospium, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof. Also disclosed herein are methods of treating a patient suffering from overactive bladder, the method comprising identifying a patient in need thereof, and administering to the patient a therapeutically effective amount of immediate release trospium, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof. Also disclosed herein are methods of alleviating a side effect of treatment for overactive bladder in a patient suffering therefrom, the method comprising identifying a patient in need thereof, and administering to the patient a therapeutically effective amount of immediate release trospium, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof.
Owner:THERAVIDA INC
Remedies for vesical hyperactivity
The present invention provides a therapeutic agent for overactive bladder comprising, as an active ingredient, a tricyclic compound represented by formula (I):[wherein R1 represents a hydrogen atom, substituted or unsubstituted lower alkyl, and the like;X1—X2—X3 represents CR5═CR6—CR7═CR8, CR5═CR6—S, and the like;Y represents —CH2S—, —SOCH2—, and the like; andR2 represents a hydrogen atom, and the like] or a pharmaceutically acceptable salt thereof.
Owner:KYOWA HAKKO KOGYO CO LTD
Combinations of imidafenacin and salivary stimulants for the treatment of overactive bladder
Disclosed are pharmaceutical compositions comprising a therapeutically effective amount of immediate release or orally disintegrating imidafenacin, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof. Also disclosed are methods of treating a patient suffering from overactive bladder, the method comprising identifying a patient in need thereof, and administering to the patient a therapeutically effective amount of immediate release or orally disintegrating imidafenacin, or pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or pharmaceutically acceptable salt thereof. Also disclosed are methods of alleviating a side effect of treatment for overactive bladder in a patient suffering therefrom, the method comprising identifying a patient in need thereof, and administering to the patient a therapeutically effective amount of immediate release or orally disintegrating imidafenacin, or pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or pharmaceutically acceptable salt thereof.
Owner:THERAVIDA INC
Combinations of darifenacin and salivary stimulants for the treatment of overactive bladder
Disclosed herein are pharmaceutical compositions comprising a therapeutically effective amount of extended release darifenacin, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof. Also disclosed herein are methods of treating a patient suffering from overactive bladder, the method comprising identifying a patient in need thereof, and administering to the patient a therapeutically effective amount of extended release darifenacin, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof. Also disclosed herein are methods of alleviating a side effect of treatment for overactive bladder in a patient suffering therefrom, the method comprising identifying a patient in need thereof, and administering to the patient a therapeutically effective amount of extended release darifenacin, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof.
Owner:THERAVIDA INC
Combinations of fesoterodine and salivary stimulants for the treatment of overactive bladder
Disclosed herein are pharmaceutical compositions comprising a therapeutically effective amount of extended release fesoterodine, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof. Also disclosed herein are methods of treating a patient suffering from overactive bladder, the method comprising identifying a patient in need thereof, and administering to the patient a therapeutically effective amount of extended release fesoterodine, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof. Also disclosed herein are methods of alleviating a side effect of treatment for overactive bladder in a patient suffering therefrom, the method comprising identifying a patient in need thereof, and administering to the patient a therapeutically effective amount of extended release fesoterodine, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof.
Owner:THERAVIDA INC
Combinations of trospium and salivary stimulants for the treatment of overactive bladder
Disclosed herein are pharmaceutical compositions comprising a therapeutically effective amount of immediate release trospium, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof. Also disclosed herein are methods of treating a patient suffering from overactive bladder, the method comprising identifying a patient in need thereof, and administering to the patient a therapeutically effective amount of immediate release trospium, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof. Also disclosed herein are methods of alleviating a side effect of treatment for overactive bladder in a patient suffering therefrom, the method comprising identifying a patient in need thereof, and administering to the patient a therapeutically effective amount of immediate release trospium, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof.
Owner:THERAVIDA INC
Combinations of trospium and salivary stimulants for the treatment of overactive bladder
Disclosed herein are pharmaceutical compositions comprising a therapeutically effective amount of extended release trospium, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof. Also disclosed herein are methods of treating a patient suffering from overactive bladder, the method comprising identifying a patient in need thereof, and administering to the patient a therapeutically effective amount of extended release trospium, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof. Also disclosed herein are methods of alleviating a side effect of treatment for overactive bladder in a patient suffering therefrom, the method comprising identifying a patient in need thereof, and administering to the patient a therapeutically effective amount of extended release trospium, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof.
Owner:THERAVIDA INC
Therapeutic Agents For Overactive Bladder
The present invention provides a therapeutic agent for overactive bladder, which has both a KATP channel opening action and a nitrate-like action and is further expected to produce an ameliorating effect for ensuring a reflexive and suitable cooperation between contraction-relaxation of abdominal / bladder wall muscle and relaxation-contraction movement of urethral sphincter during urination and urine storage, so that the therapeutic agent does not induce oliguria or urinary retention and has no serious side effect. Nicorandil or a pharmaceutically acceptable salt thereof is used as an active ingredient.
Owner:CHUGAI PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com