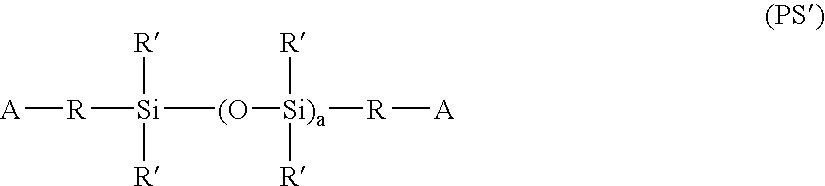Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
43 results about "Ophthalmological implant" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
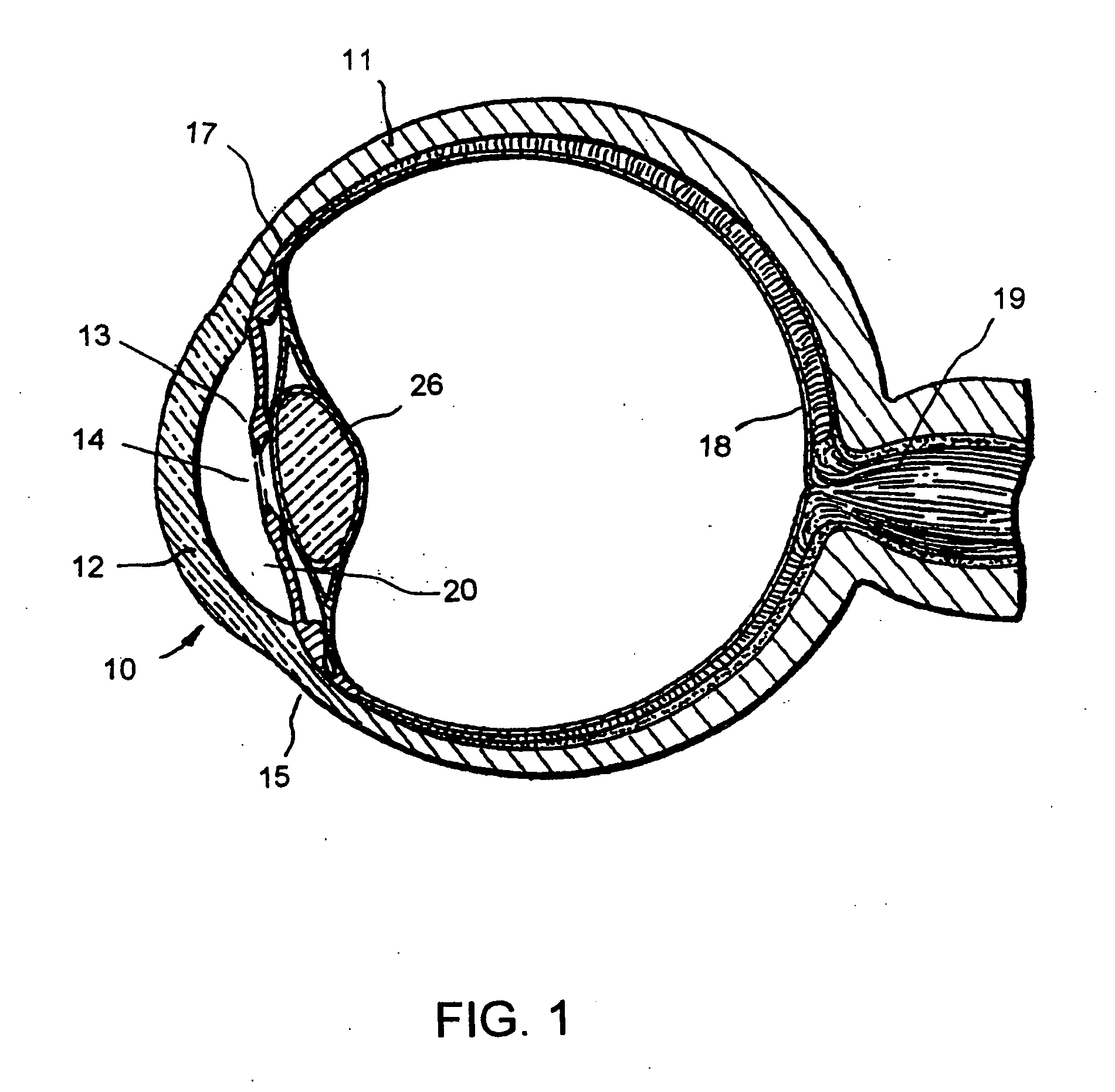
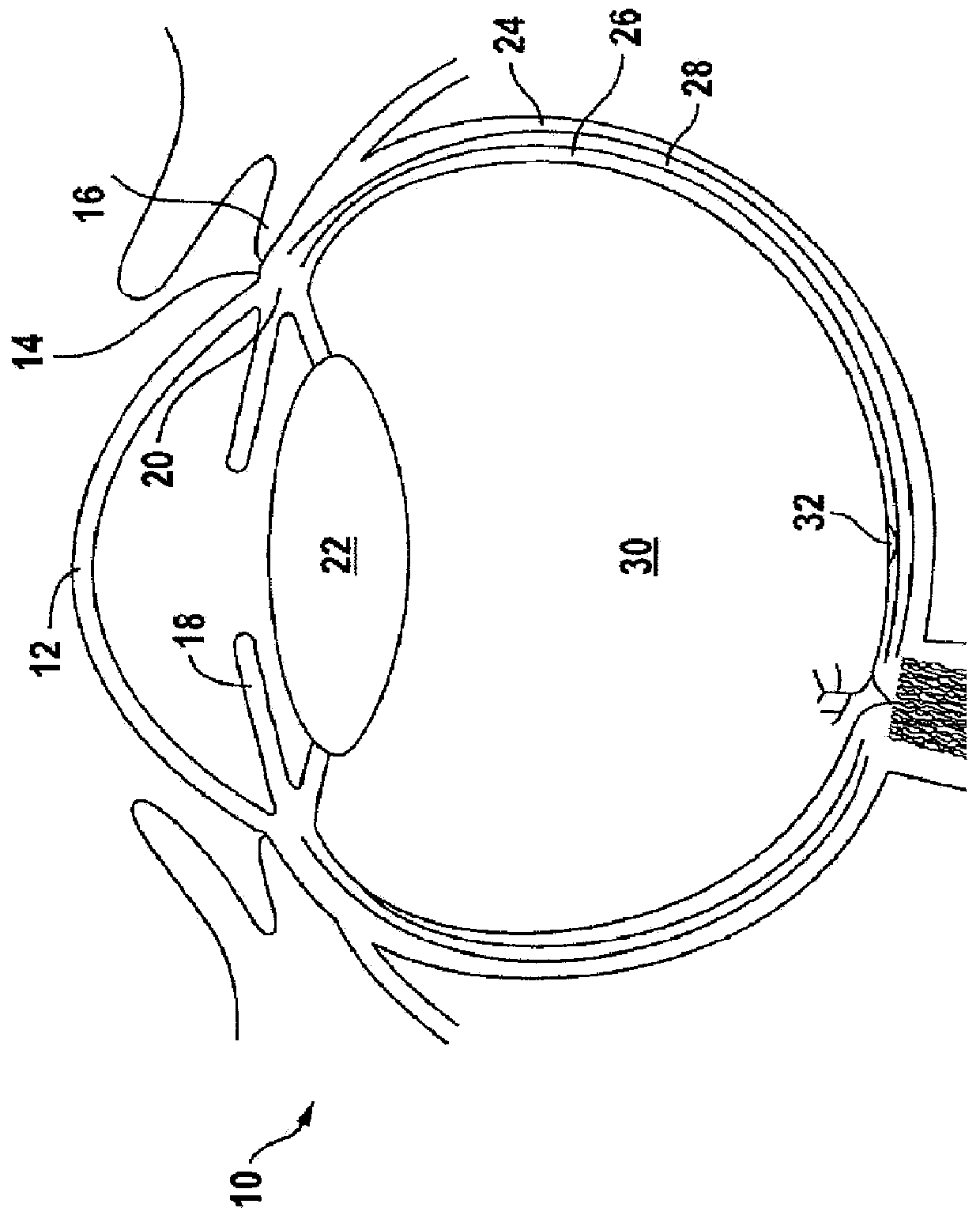
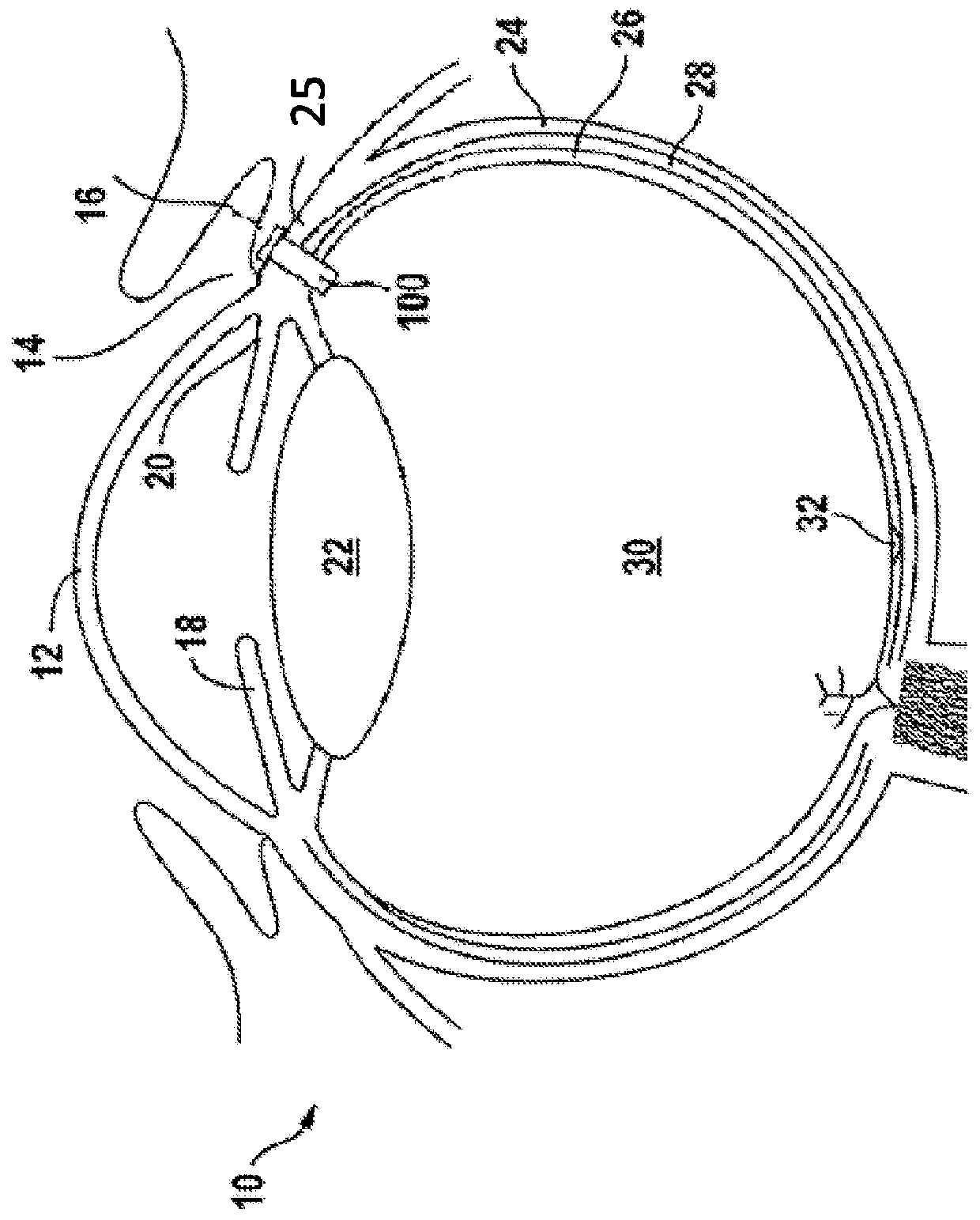
Ophthalmology implants and methods of manufacture
The present disclosure provides an ophthalmology implant and methods for treating glaucoma or optic neural transmission deficiency, wherein at least a portion of the implant is made of or includes a nanometer-sized substance, such as nanotubes, nanofibers, sheets from nanotubes, nanowires, nanofibrous mesh and the like.
Owner:GLAUKOS CORP
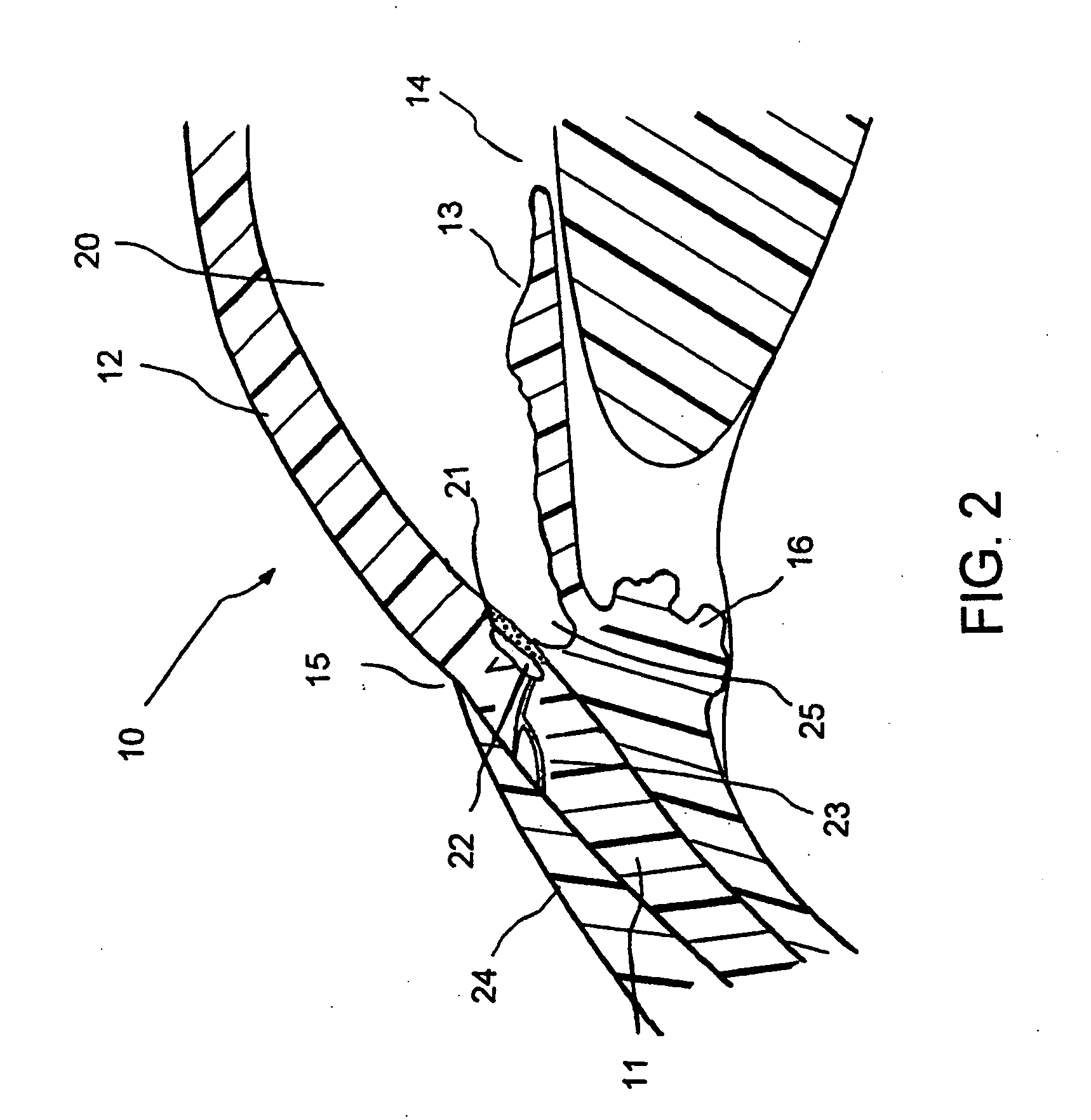
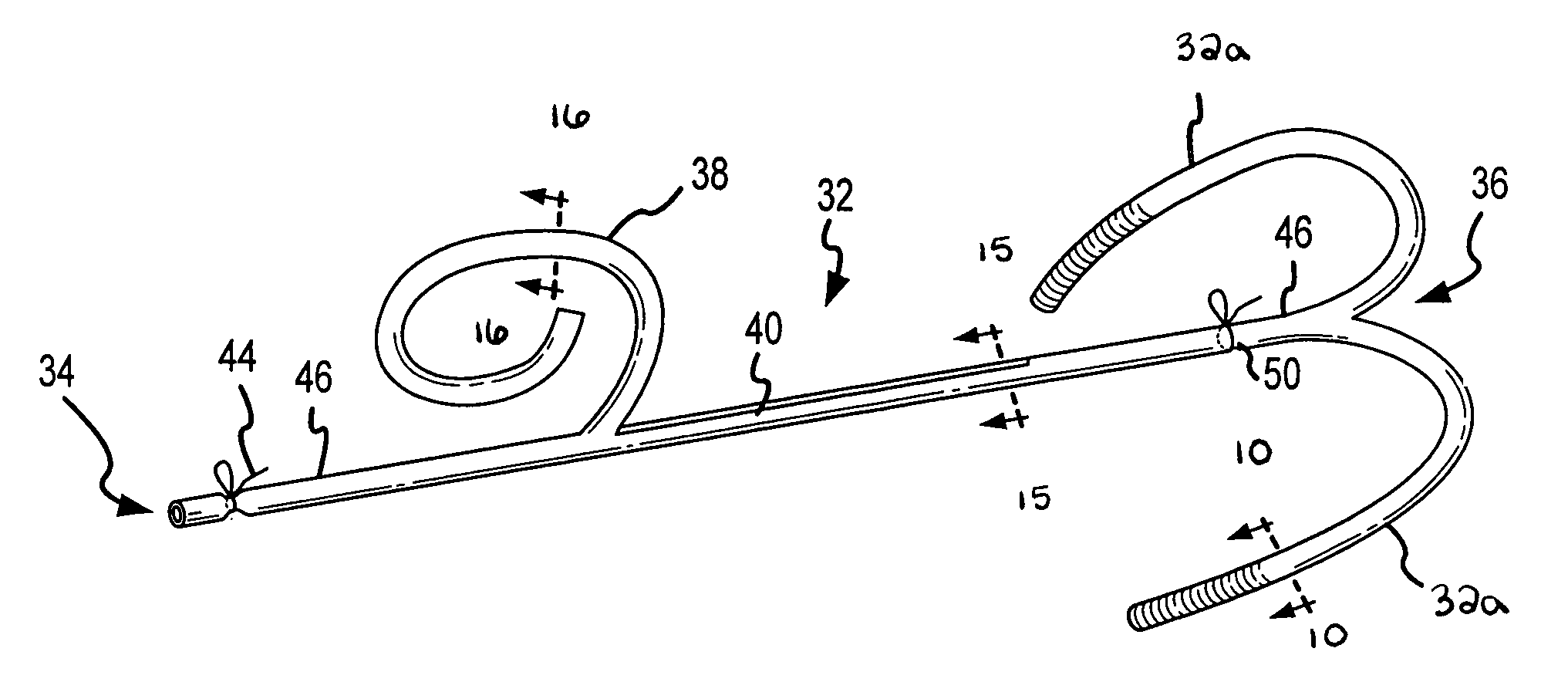
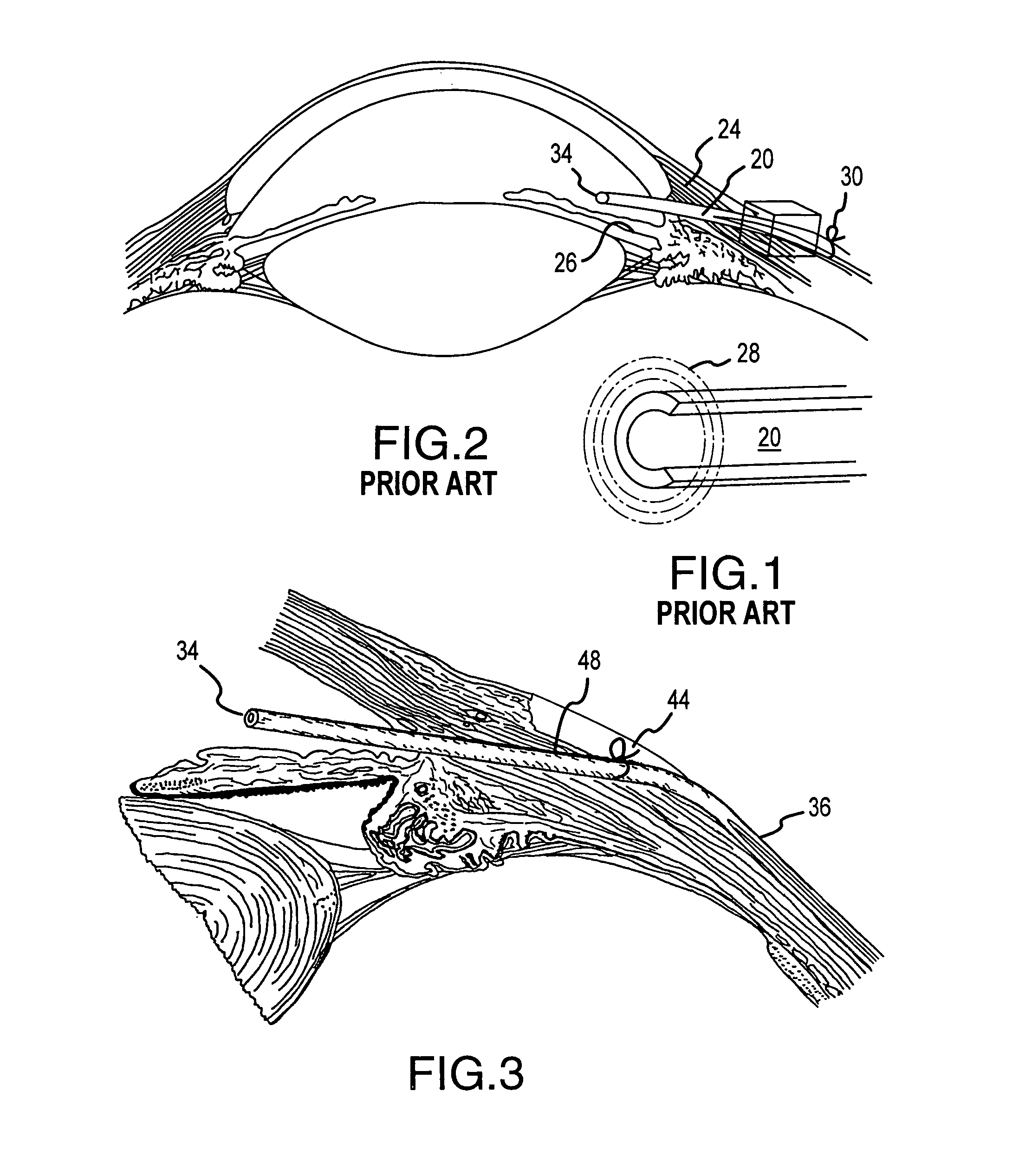
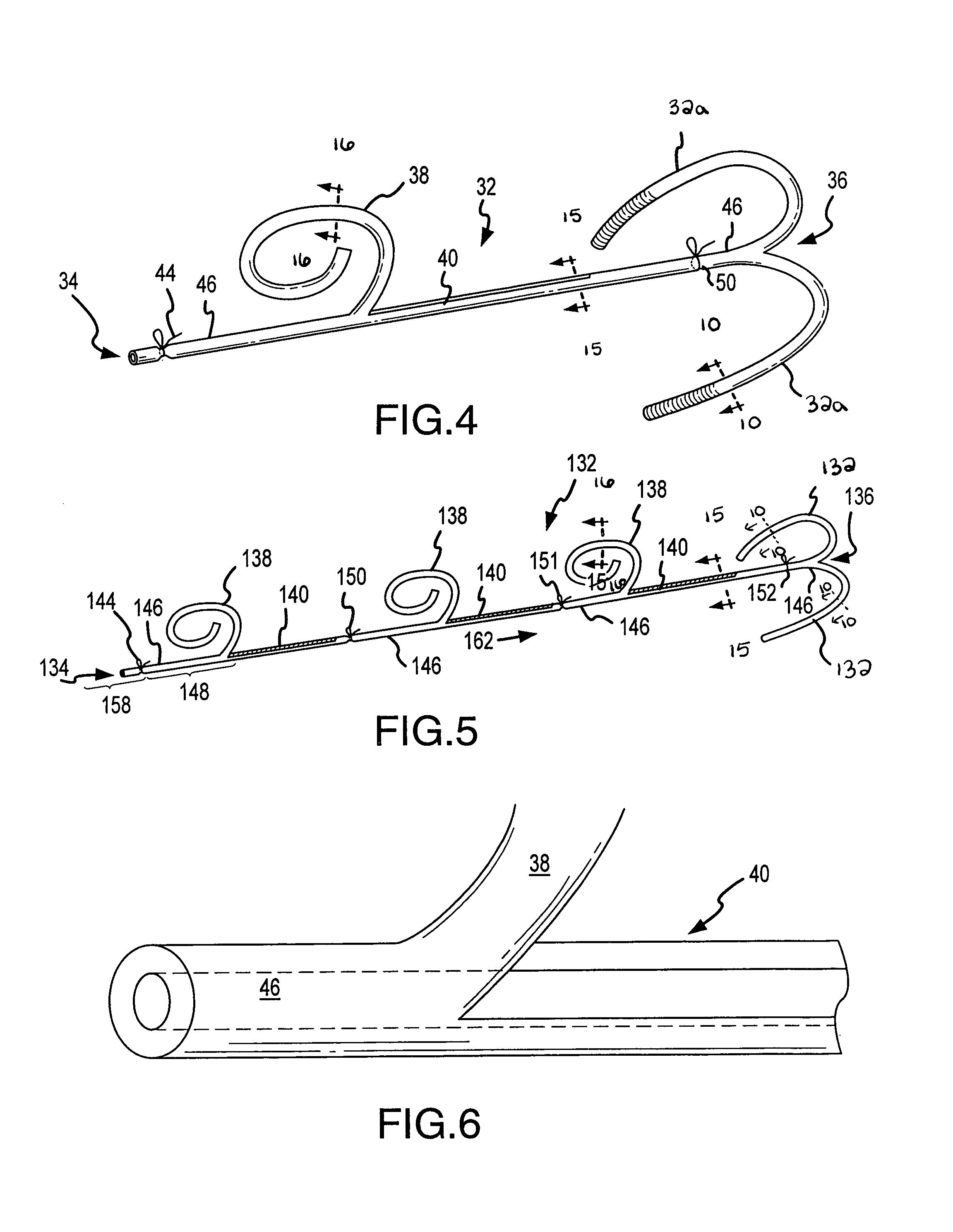
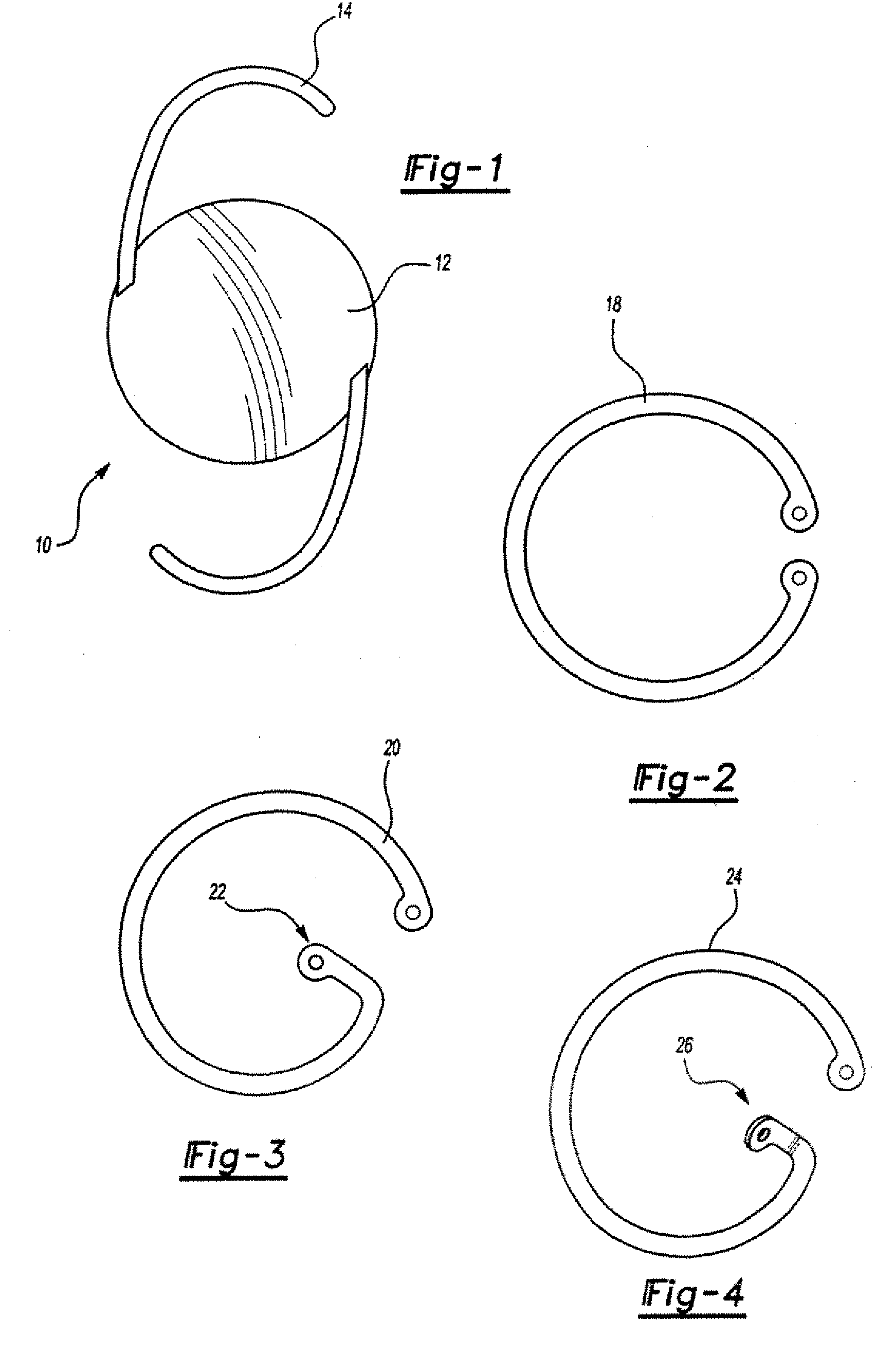
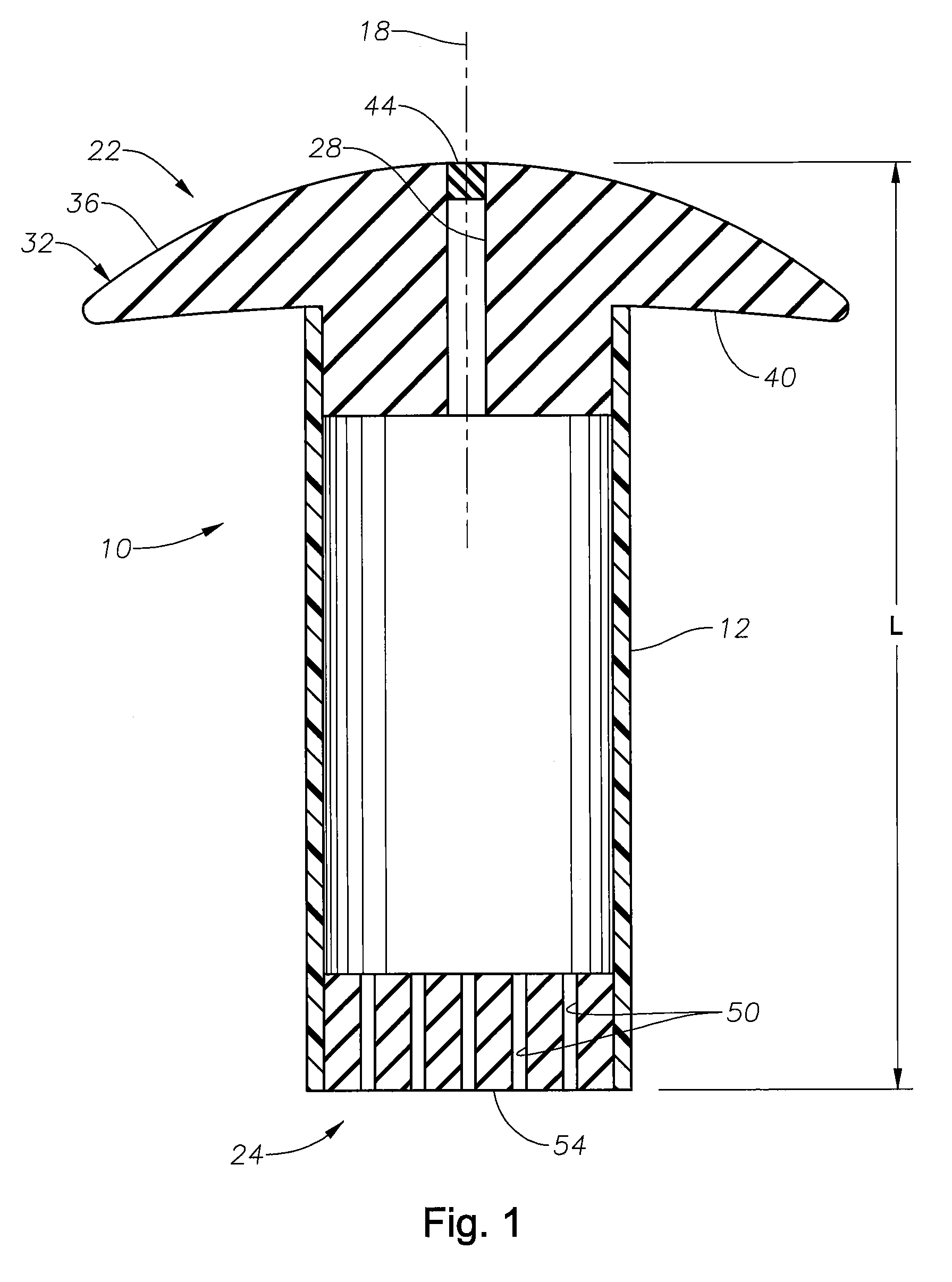
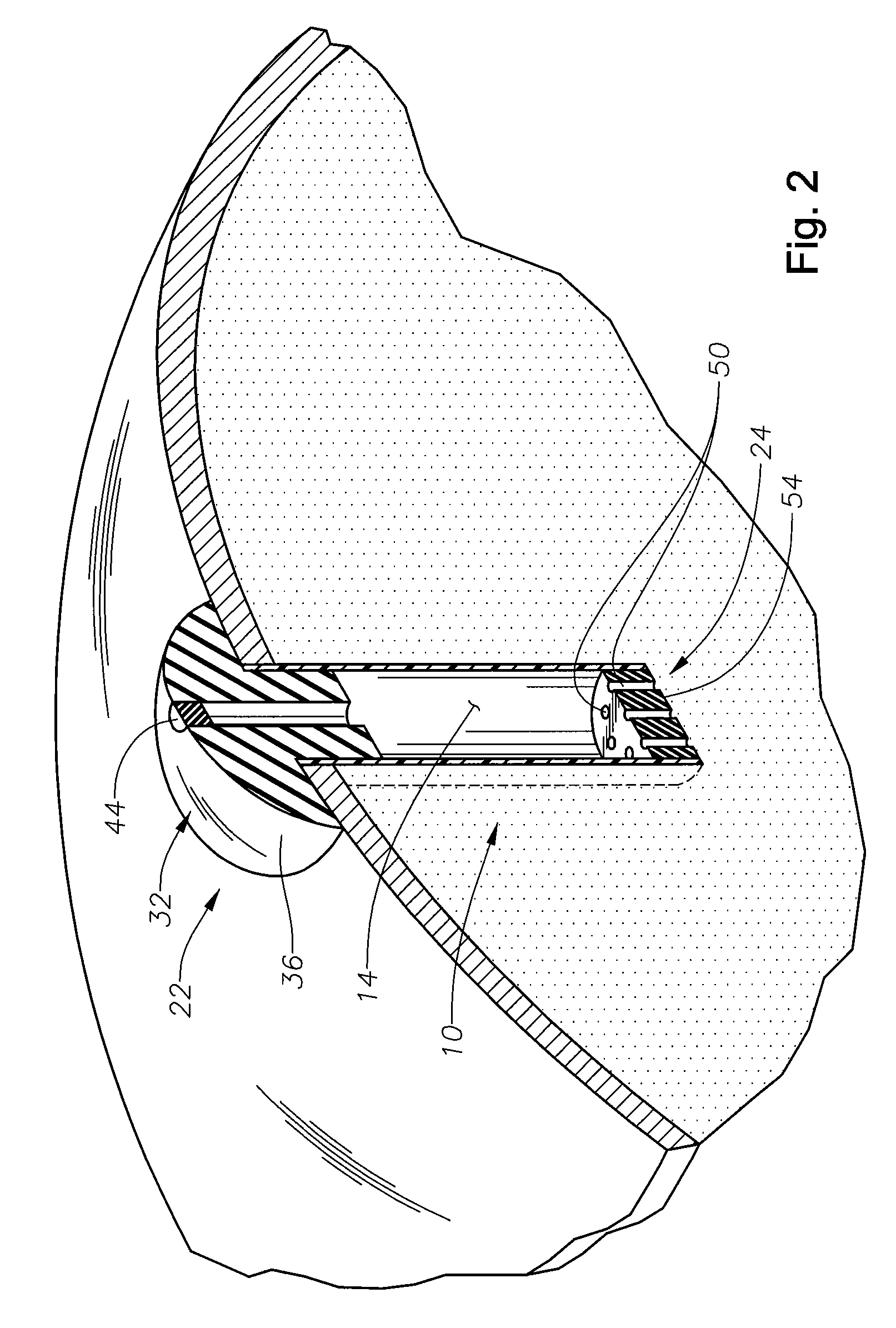
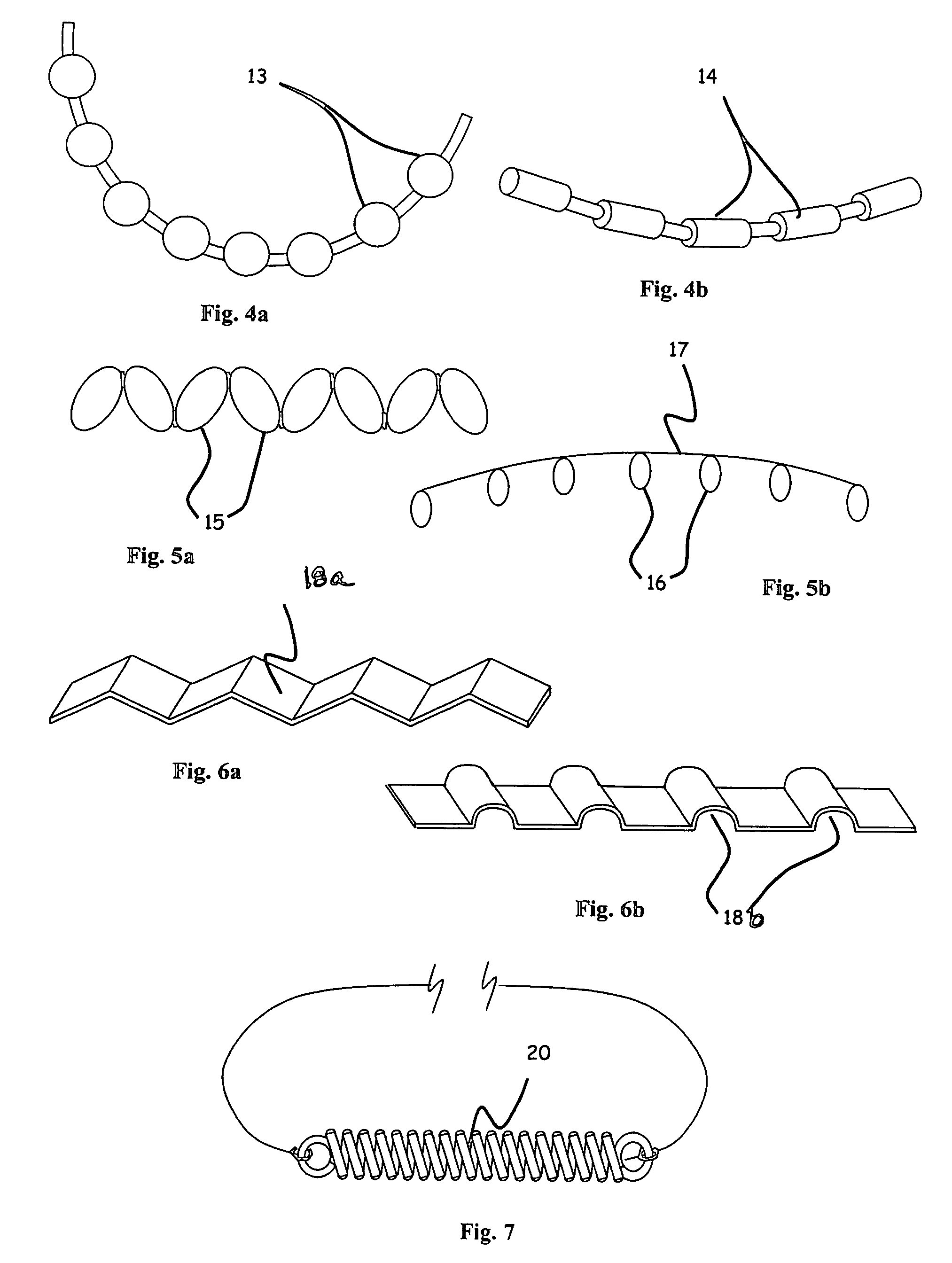
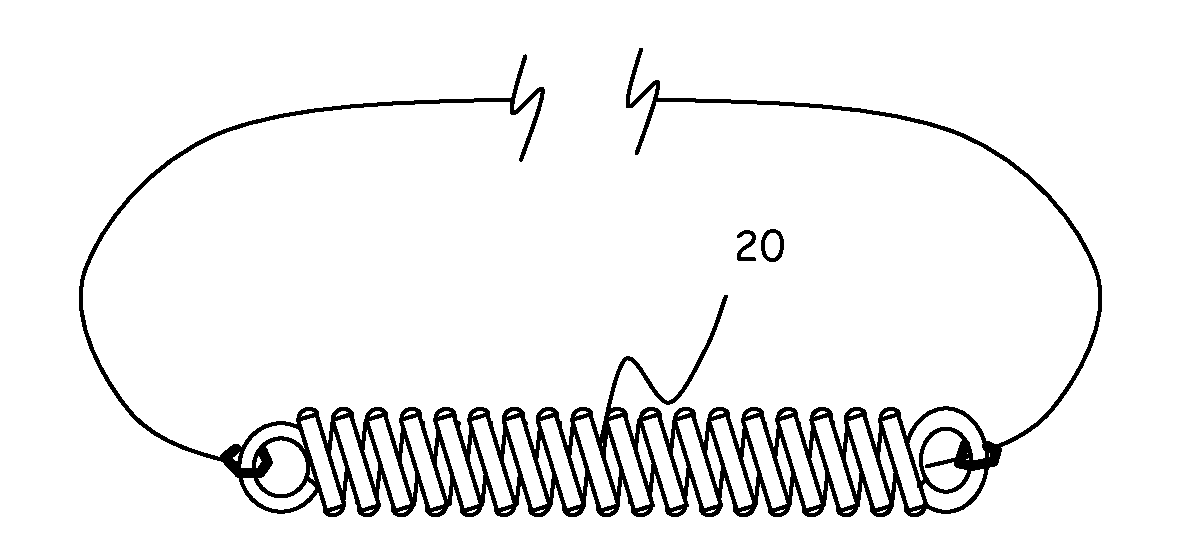
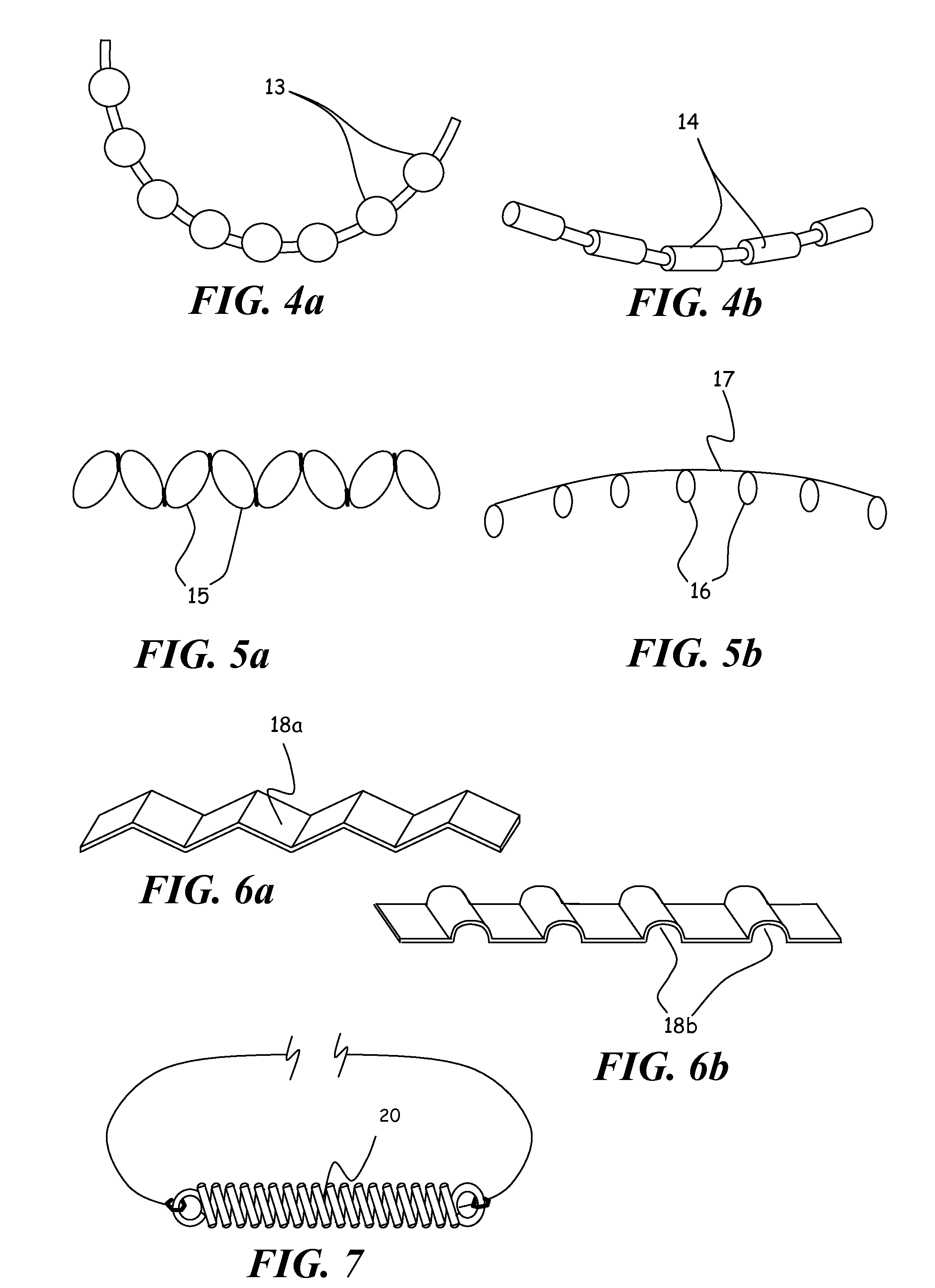
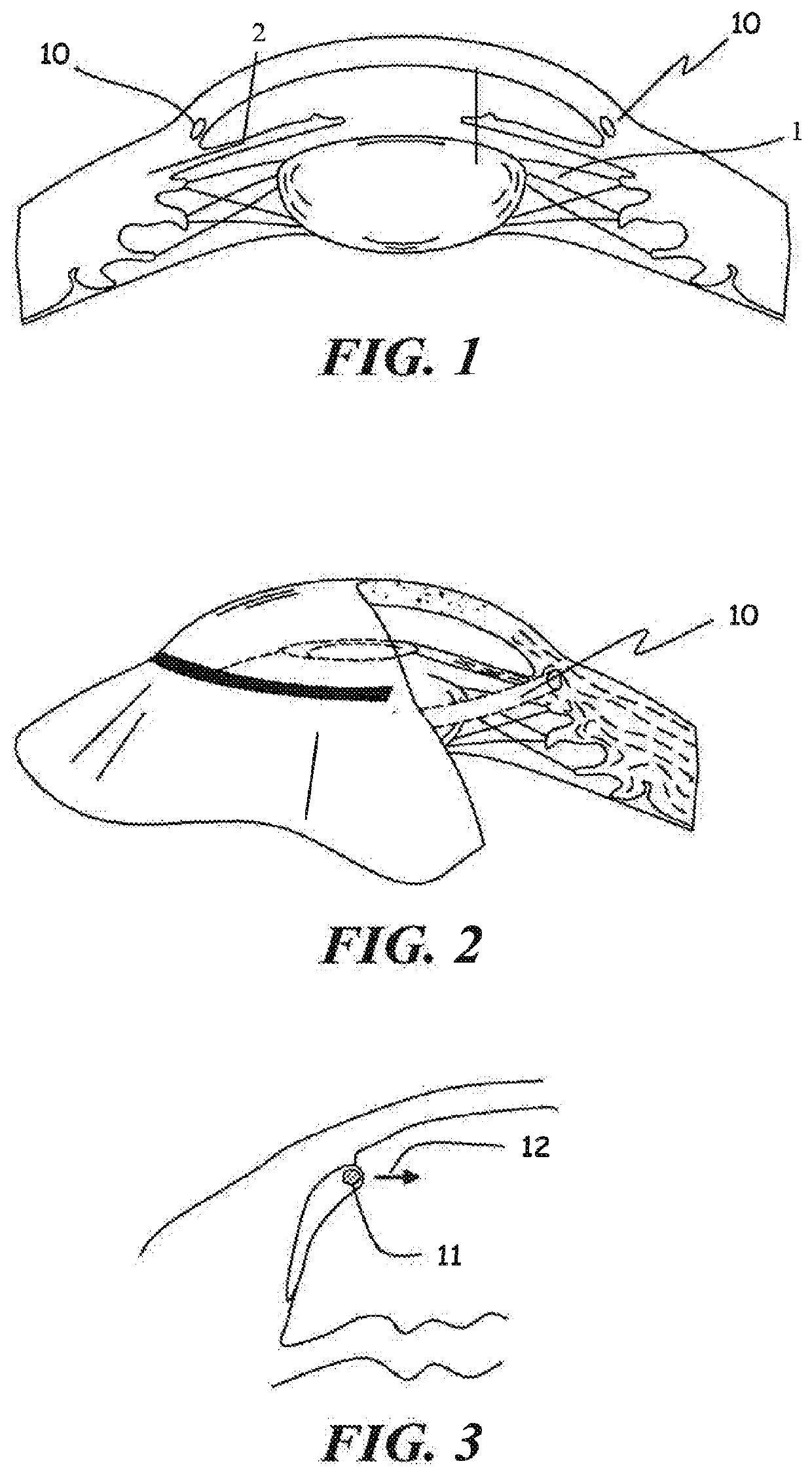
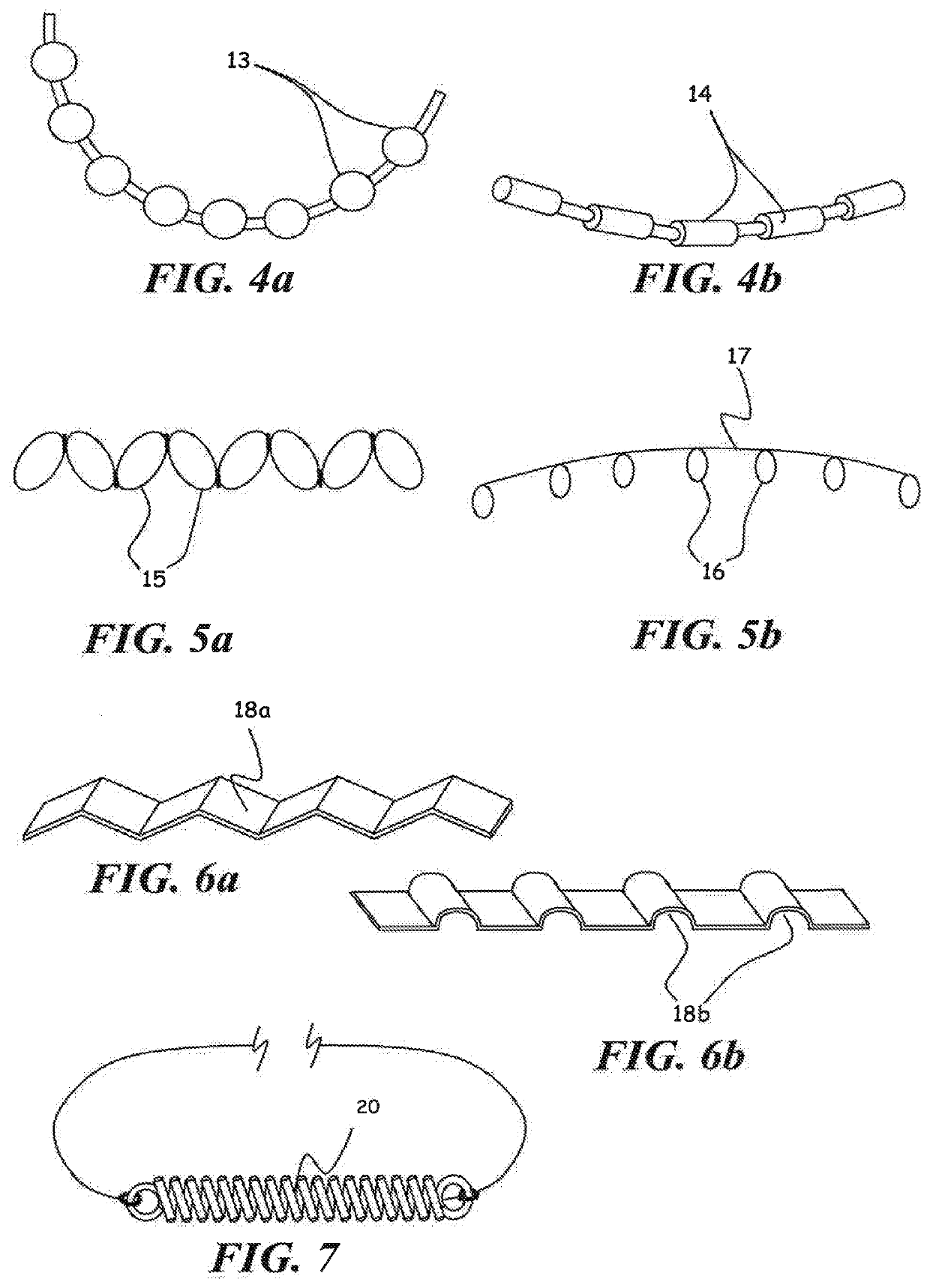
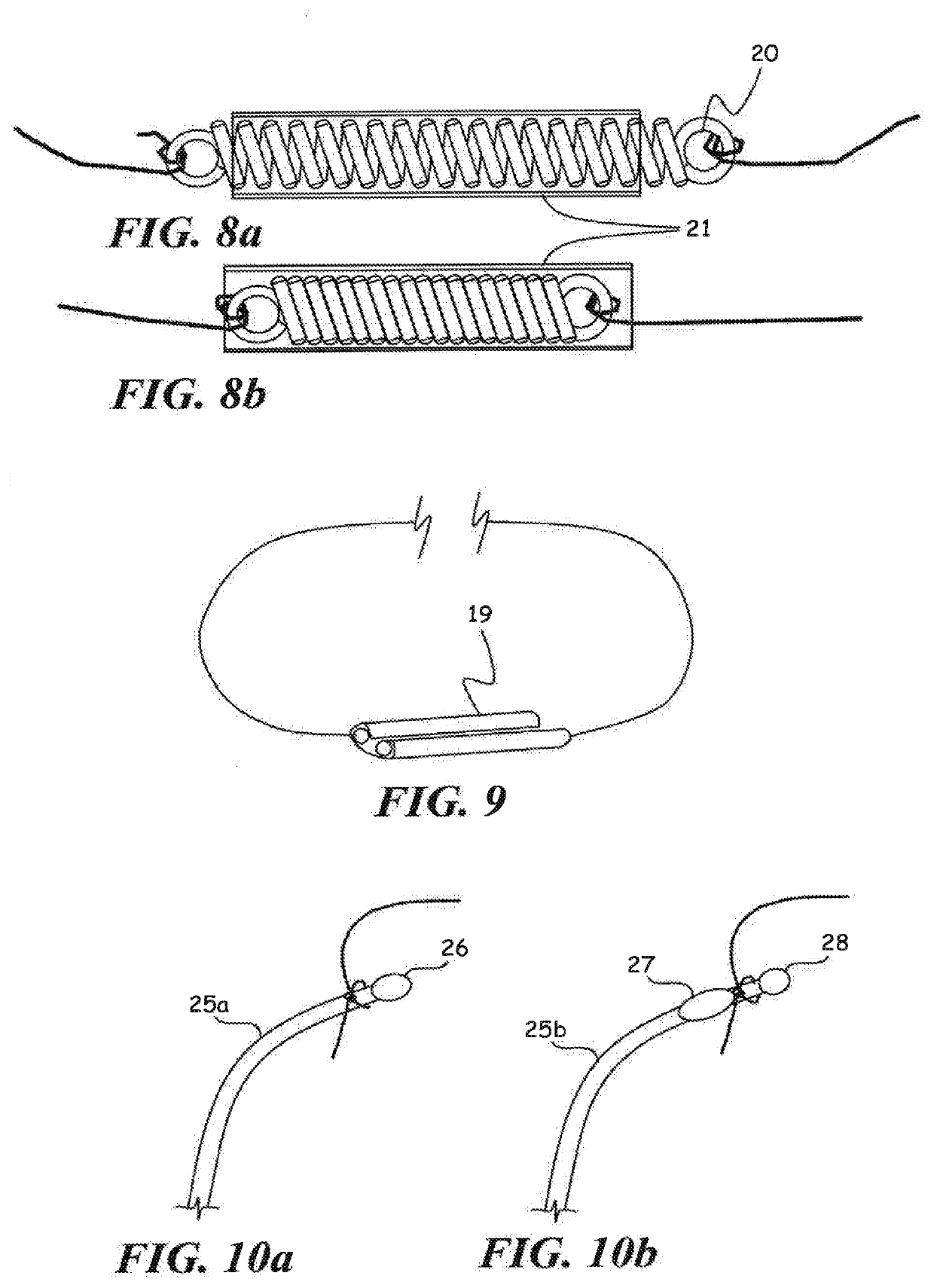
C-shaped cross section tubular ophthalmic implant for reduction of intraocular pressure in glaucomatous eyes and method of use
InactiveUS6962573B1Inhibit migrationReduction in bleb diameterEye implantsEar treatmentOphthalmological implantAqueous humor
A tube for implantation into the eye for replacement conduction of aqueous humor from the chambers of the eyeball to the subconjunctival tissue and ultimately to the venous system is comprised of an elongated fluid conducting conduit having distal and proximate ends, a sidewall and an interior passageway and at least one longitudinally extending opening in the sidewall that exposes the interior passageway and at least one nidi-forming structure carried by the conduit and extending laterally therefrom to implement the formation of at least one aqueous filtration bleb in the tissue of the eyeball. In one embodiment, the tube also contains at least one releasable ligature circumscribing the conduit. In another embodiment, the tube also contains an anchor appended to the conduit to prevent it from migrating from its placement site.
Owner:AQ BIOMED LLC
Ophthalmic implant for treatment of glaucoma
ActiveUS20060195187A1Increase outflowGood fluid permeabilityEye implantsEye surgeryOphthalmological implantSchlemm's canal
An implant is placed within Schlemm's canal of the eye and provides tension to the trabecular meshwork. The tension is continuous and increases the aqueous outflow without the necessity of administering cholinergic drugs to treat glaucoma.
Owner:NOVA EYE INC
Ocular Implants Deployed in Schlemm's Canal of the Eye
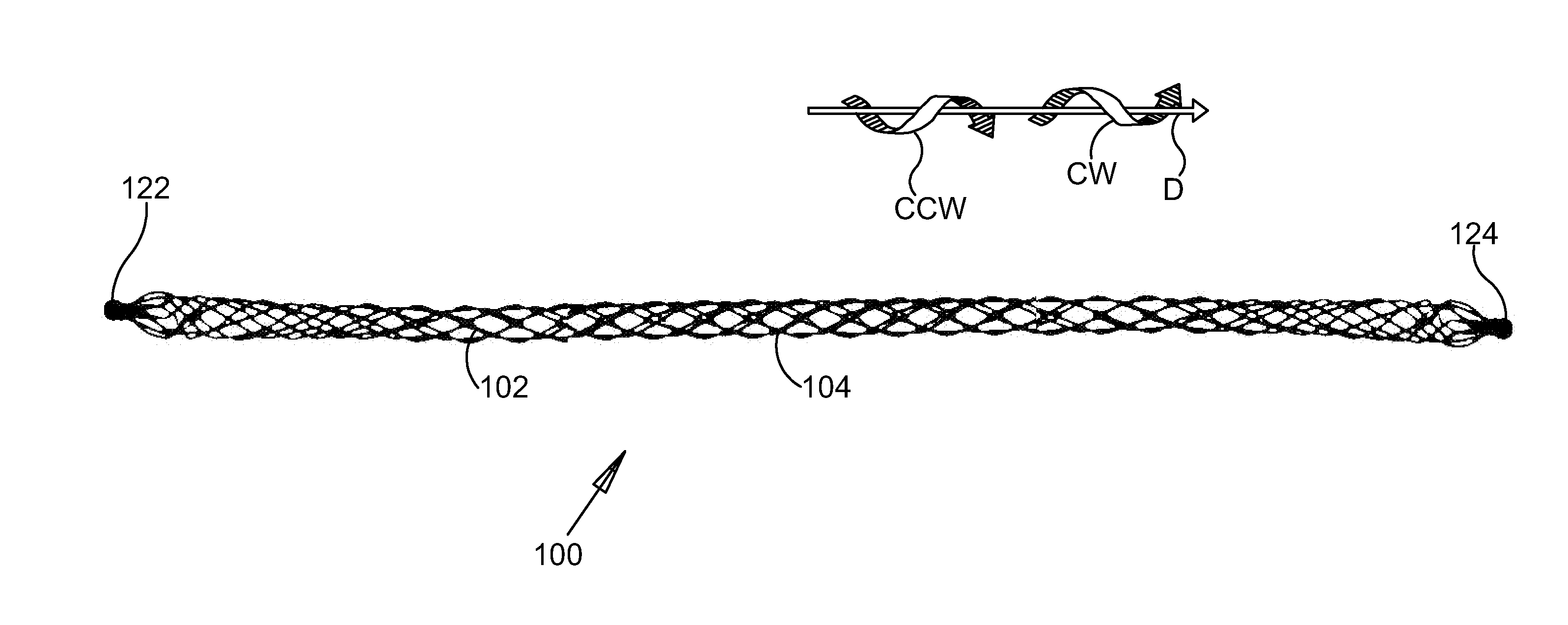
ActiveUS20110319806A1Inhibit migrationEasy to moveEye surgeryIntravenous devicesOphthalmological implantSchlemm's canal
An ocular implant is provided that is adapted to reside at least partially in a portion of Schlemm's canal of an eye. The implant includes an implant body having plurality of filars extending along a longitudinal axis of the implant. The implant is configured to move between a radially collapsed state and a radially expanded state. In some embodiments, the implant is configured to assume the radially expanded state when no external forces are acting thereon. Methods of delivering and using the ocular implant are also provided.
Owner:ALCON INC
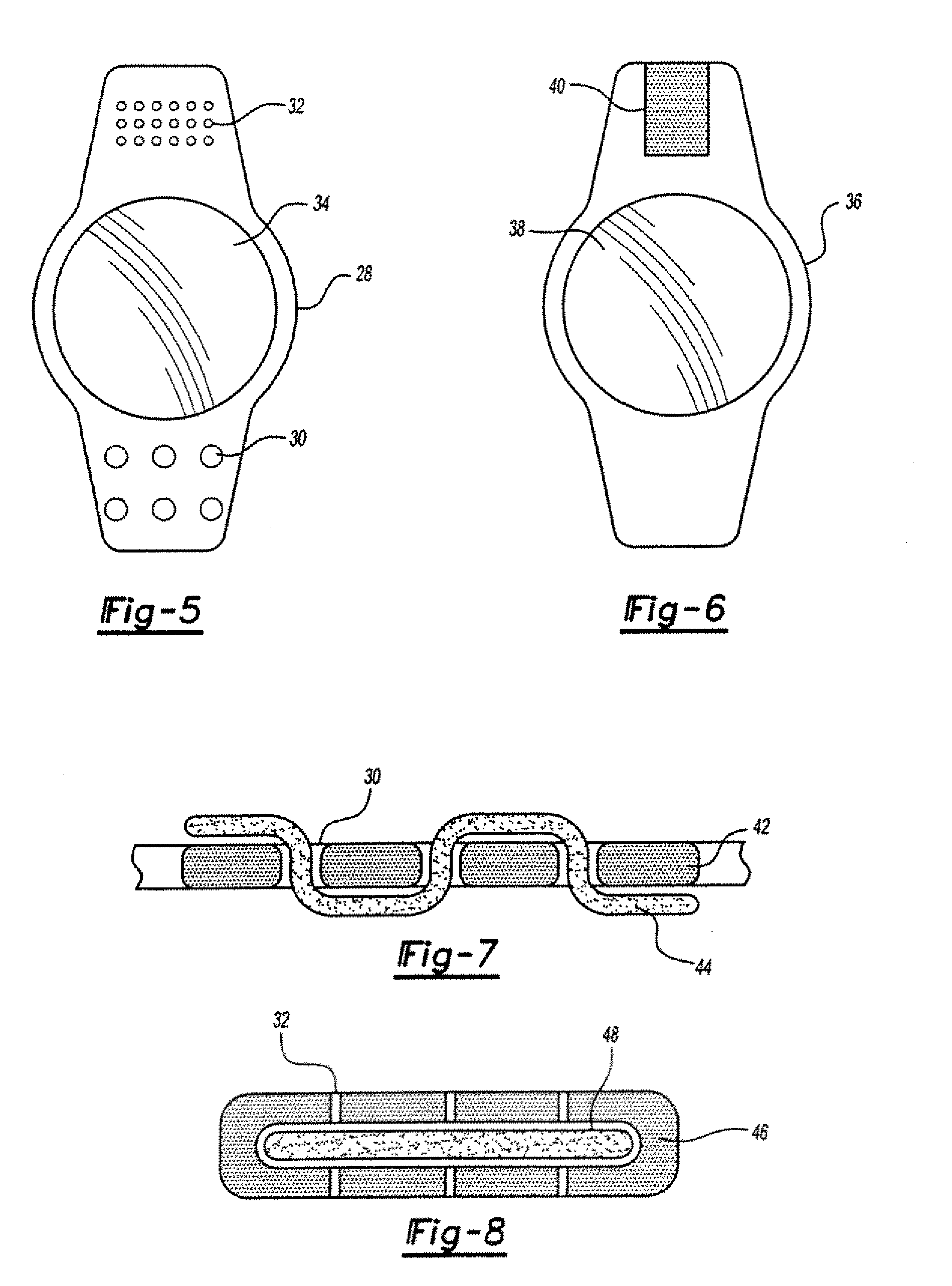
Sustained release ophthalmological device and method of making and using the same
InactiveUS20060246112A1Low costReduce needPharmaceutical delivery mechanismEye treatmentOphthalmological deviceOphthalmological implant
An ophthalmological implant including a layer of pharmaceutical agent and an overlying layer of a bioerodible material, a biodegradable material a bioavailable material or a mixture thereof.
Owner:SNYDER MICHAEL E +1
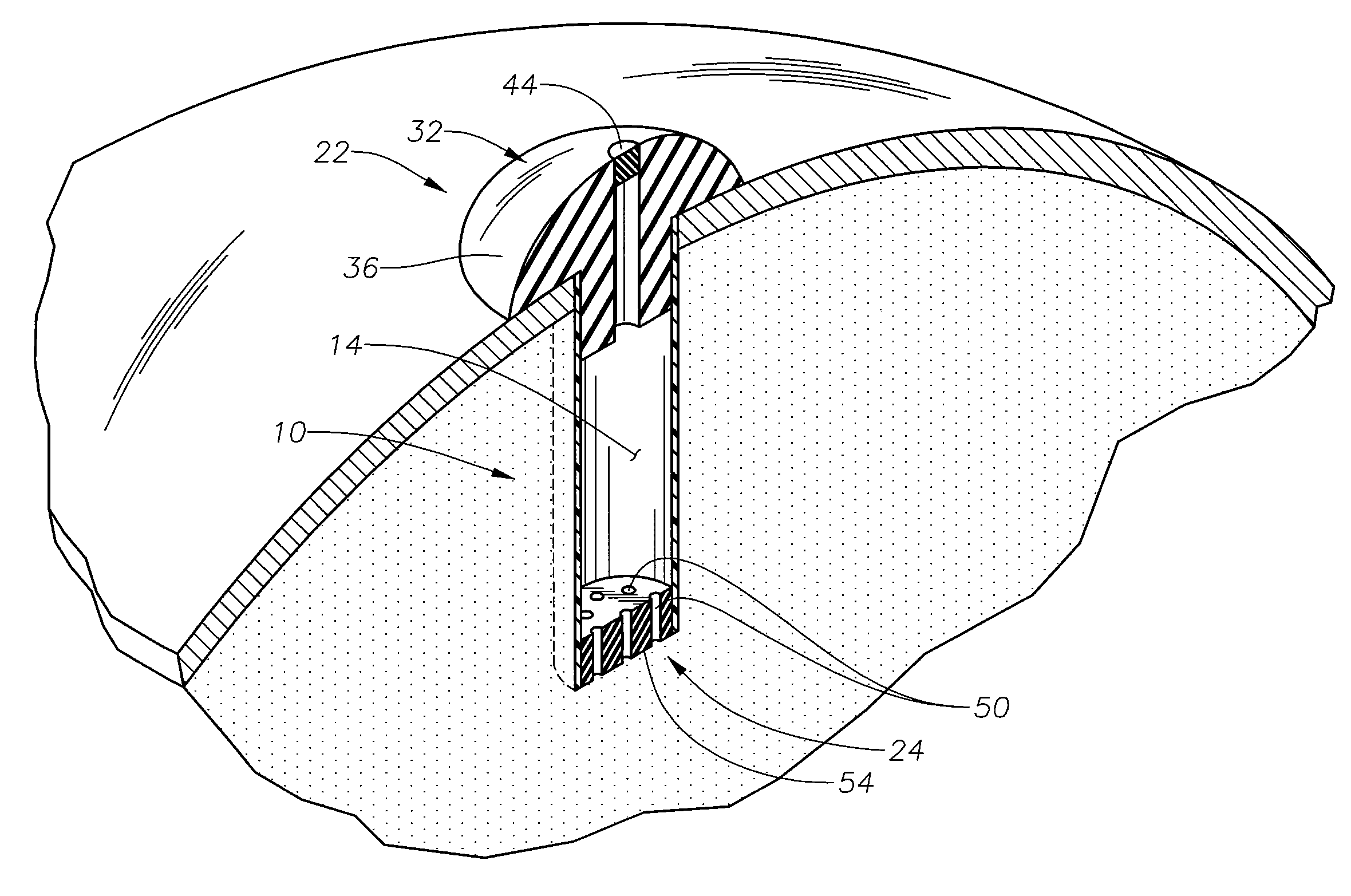
In-situ refillable ophthalmic implant
InactiveUS20100174272A1Control releaseEye implantsMedical devicesOphthalmological implantControl release
The present invention is directed to an in-situ refillable ophthalmic implant having a refill port in communication with a reservoir and a release control mechanism. The present invention also relates to methods of forming and using the ophthalmic implant. Preferably, the control release mechanism include opening[s] providing for passive passage of pharmaceutical ophthalmic composition, particularly therapeutic agent, out of the reservoir, through the opening[s] and into the eye.
Owner:ALCON RES LTD
Ophthalmic implant for treatment of glaucoma
ActiveUS8034105B2Sufficient forceImprove permeabilityEye implantsEye surgeryOphthalmological implantSchlemm's canal
A method is provided for increasing the outflow of fluid through Schlemm's canal that is useful for treatment of glaucoma. The implant is placed in Schlemm's canal by use of a flexible delivery instrument attached to the implant. The instrument and implant are positioned within the canal, the implant is released and the distal and proximal ends of the implant are connected to apply sufficient axial tensioning force on the inner wall of the canal to increase fluid permeability. In another embodiment a delivery instrument attached to the implant is positioned in the canal securing one of the distal or proximal ends of the implant within the canal. The implant provides sufficient axial tensioning force on the inner wall of the canal to increase fluid permeability of the inner wall of the canal. The other of the distal or proximal ends may be secured to maintain the tensioning force on the inner wall of the canal.
Owner:NOVA EYE INC
Ophthalmic implant for treatment of glaucoma
InactiveUS20120010702A1Sufficient forceImprove permeabilityEye implantsEye surgeryOphthalmological implantSchlemm's canal
An implant is placed within Schlemm's canal of the eye and provides tension to the trabecular meshwork. The tension is continuous and increases the aqueous outflow without the necessity of administering cholinergic drugs to treat glaucoma.
Owner:NOVA EYE INC
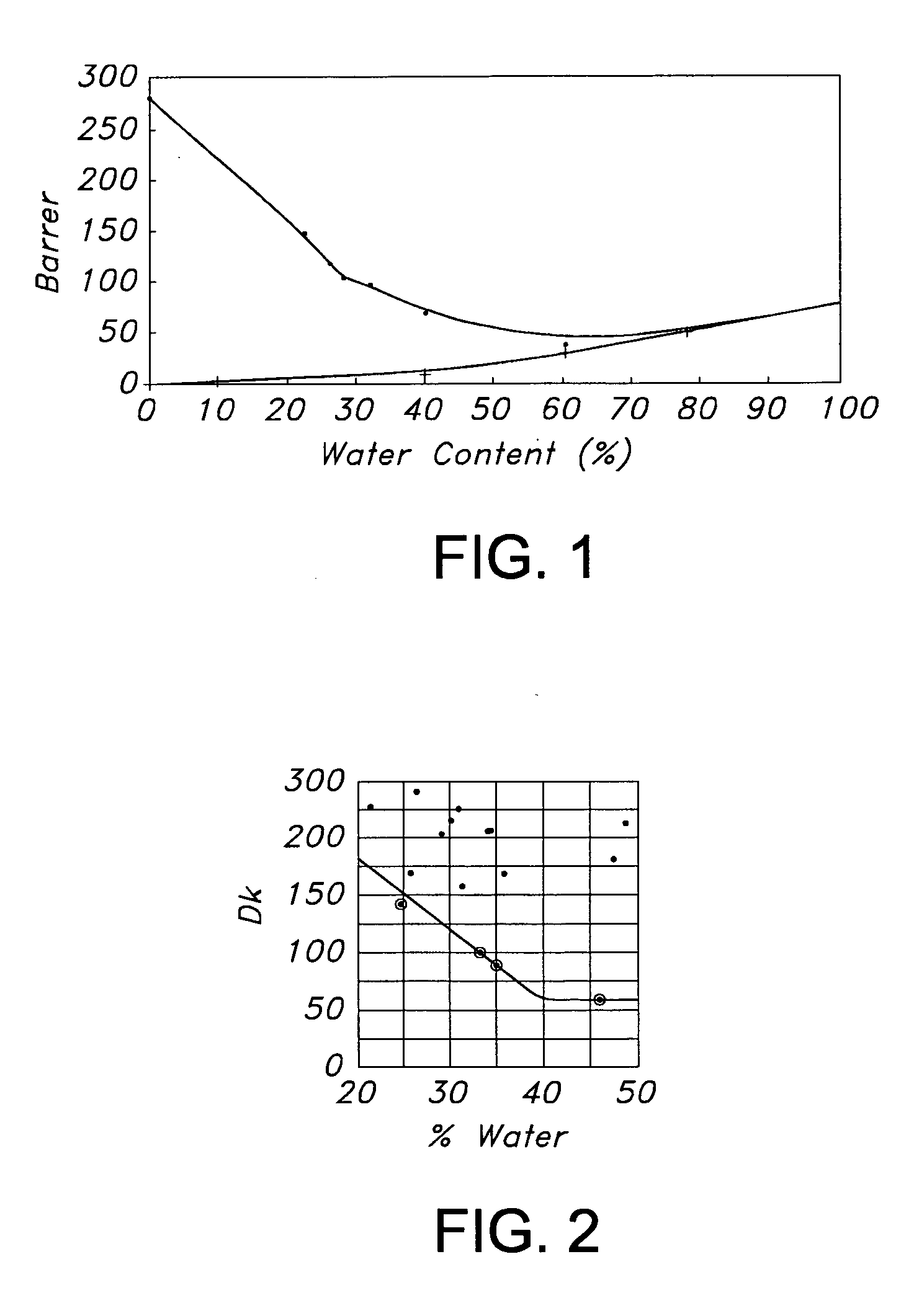
Hydrogel copolymers for biomedical devices
InactiveUS20060142525A1High oxygen permeabilityDesirable modulusOptical partsProsthesisOphthalmological implantBiomedical engineering
Hydrogel copolymers are useful for forming biomedical devices, particularly ophthalmic devices including contact lenses, intraocular lenses and ophthalmic implants. The copolymers have a desirable combination of oxygen permeability, tensile modulus, and water content, especially for soft contact lenses.
Owner:BAUSCH & LOMB INC
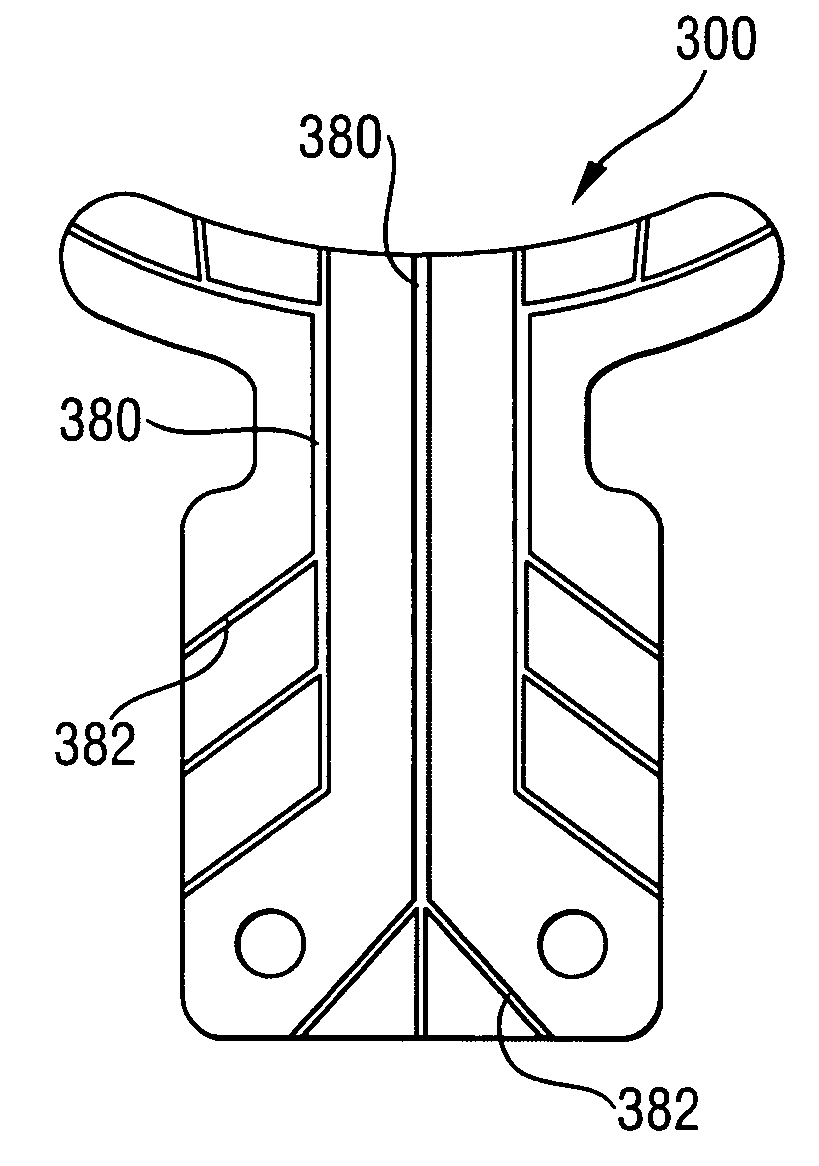
Article and method for ocular aqueous drainage
ActiveUS7160264B2Lower eye pressurePromote increased drainageEye implantsEye surgeryOphthalmological implantBiomedical engineering
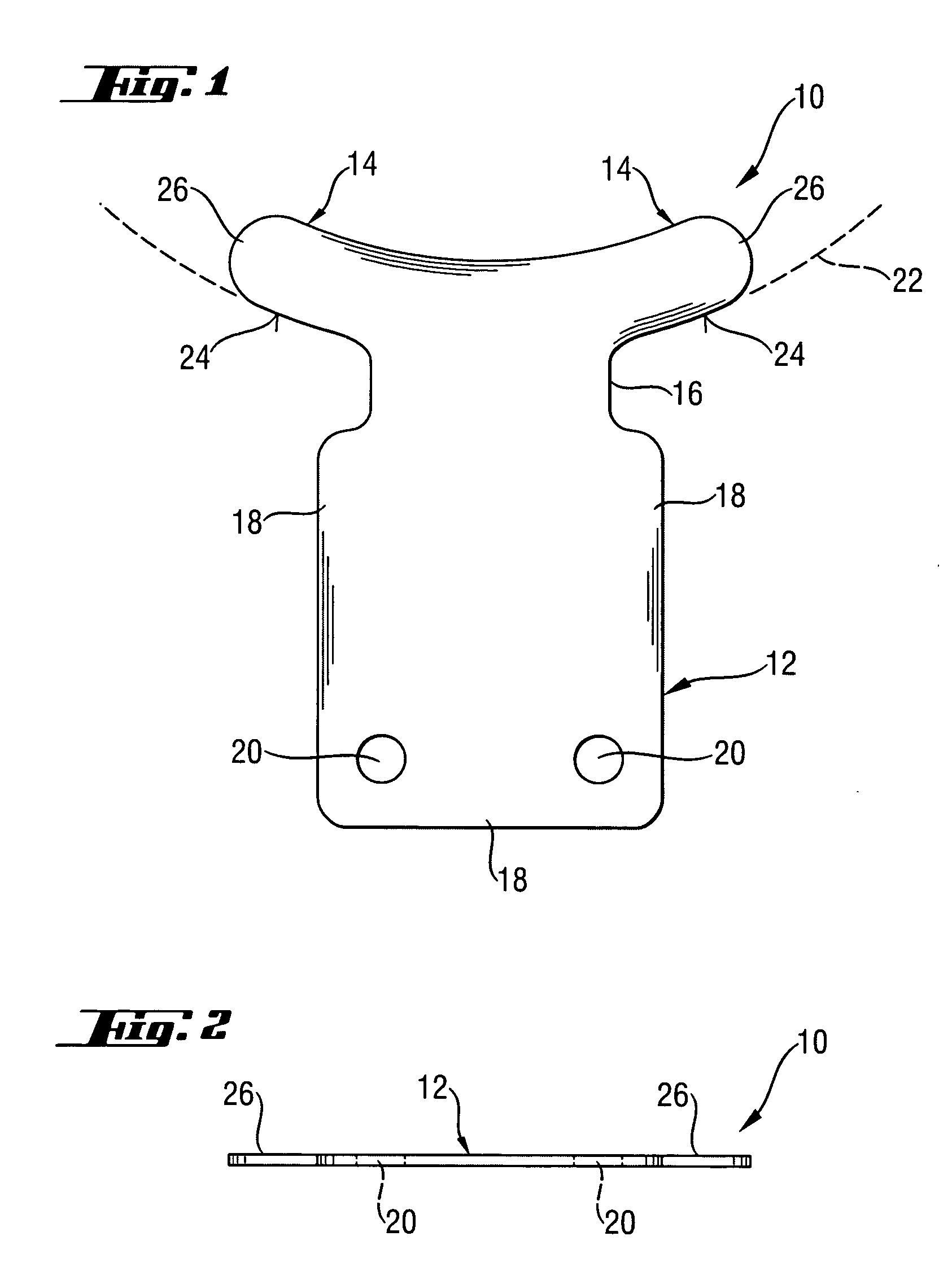
An ophthalmic implant having a body for positioning in a recess created in the sclera, feet for positioning in the anterior chamber, and a neck between the body and the feet for positioning in a scleral opening created between the scleral recess and the anterior chamber. The implant feet extend beyond the implant body and have a curvature approximating the curvature of the anterior chamber. The implant has drainage passageways for draining ocular fluid from the anterior chamber. The passageways are formed in outer surfaces or through the interior of the implant. Interior passageways are formed in surfaces of layers of the implant or by voids in inner layers of the implant. Related methods include creating undercuts that receive outer portions of the implant body and creating a scleral opening that is longer than the implant neck.
Owner:WOUND HEALING OF OKLAHOMA
Method of calculating the required lens power for an opthalmic implant
ActiveUS7476248B2The calculation method is accurateEye diagnosticsIntraocular lensOphthalmological implantCornea eye
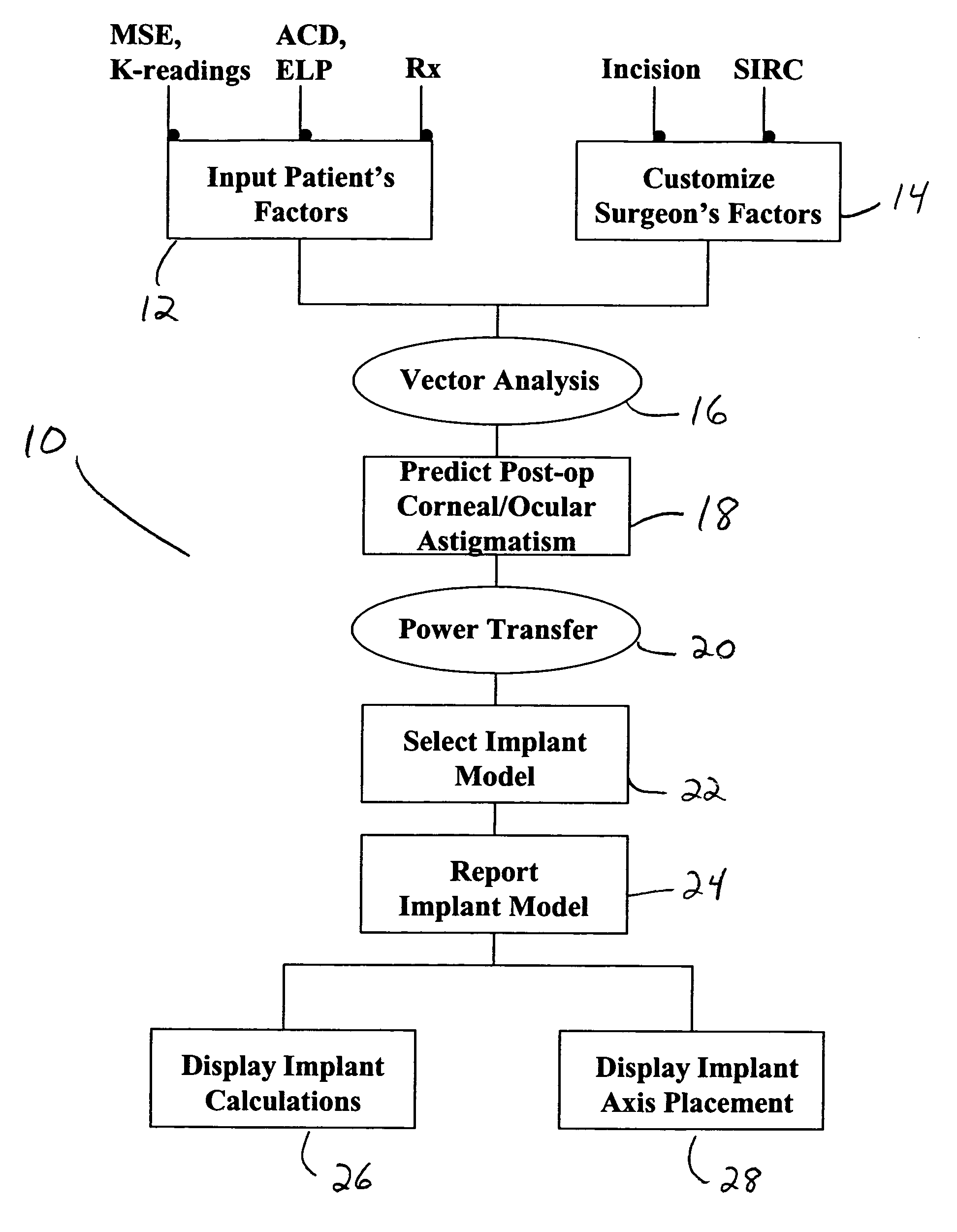
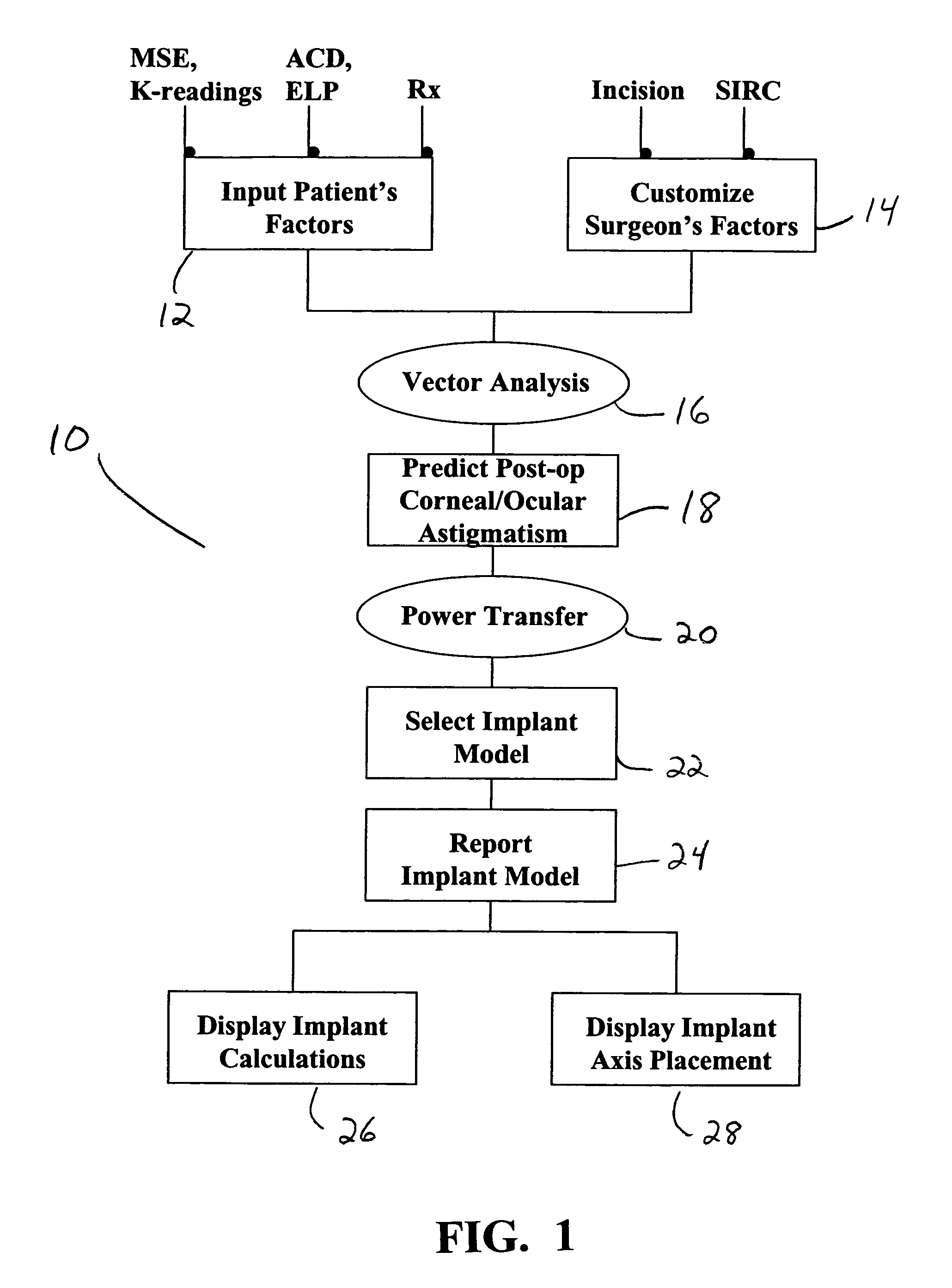
A method for calculating the required cylindrical power of a toric implant by using both the measured pre-operative corneal / ocular astigmatism and the predicted surgically-induced astigmatism. The post-operative corneal / ocular astigmatism is predicted using power vector analysis of the surgical technique employed by the surgeon. Such a method provides a more accurate method of calculating the required spherocylindrical refractive power of the implant. The method can be implemented manually, but preferably is automated by implementation on a computer through appropriate software.
Owner:ALCON INC
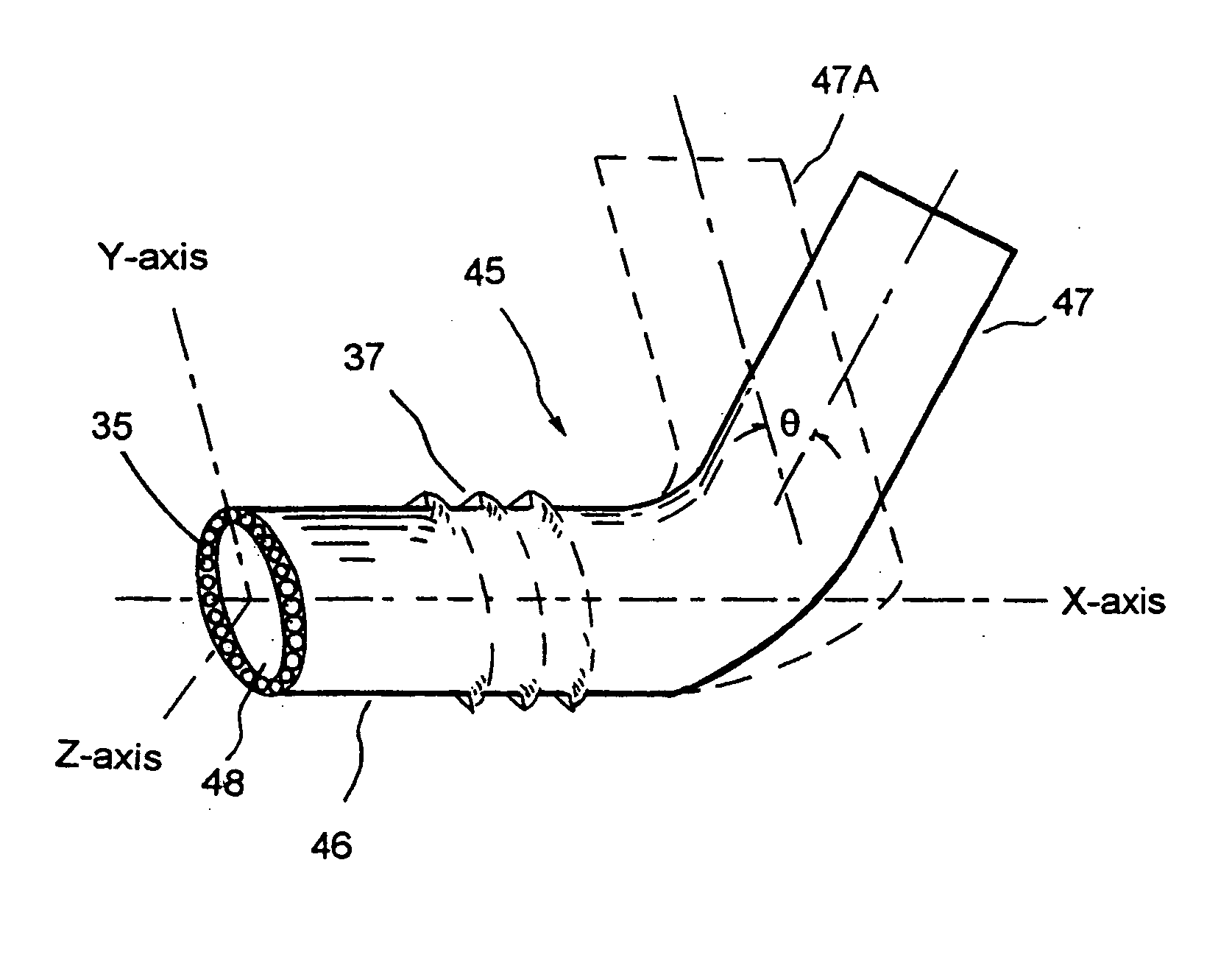
C-shaped cross section tubular ophthalmic implant for reduction of intraocular pressure in glaucomatous eyes and method of use
InactiveUS20050261624A1Lower eye pressureInhibit migrationEye implantsEye surgeryOphthalmological implantIntraocular pressure
An implant may be used for implantation into tissue of a body. The implant includes an elongated conduit and a loop. The elongated conduit has an interior passageway for conducting fluid. The loop has an interior circumference with a fluid conducting channel formed therein. The channel is interconnected with the interior passageway for delivery of fluid between the channel and the interior passageway.
Owner:AQ BIOMED LLC
Sustained release ophthalmological device and method of making and using the same
InactiveUS7090888B2Eliminate needLow costPretreated surfacesAdhesive dressingsOphthalmological implantVariable thickness
A method of making an ophthalmological implant by applying a layer of pharmaceutical agent and an overlying layer of a bioerodible material, a biodegradable material, a bloavailable material or a mixture thereof, the overlying layer having variable thickness and being dimensioned for prolonged release of the pharmaceutical agent from the implant as the overlying layer degrades.
Owner:SNYDER MICHAEL E +1
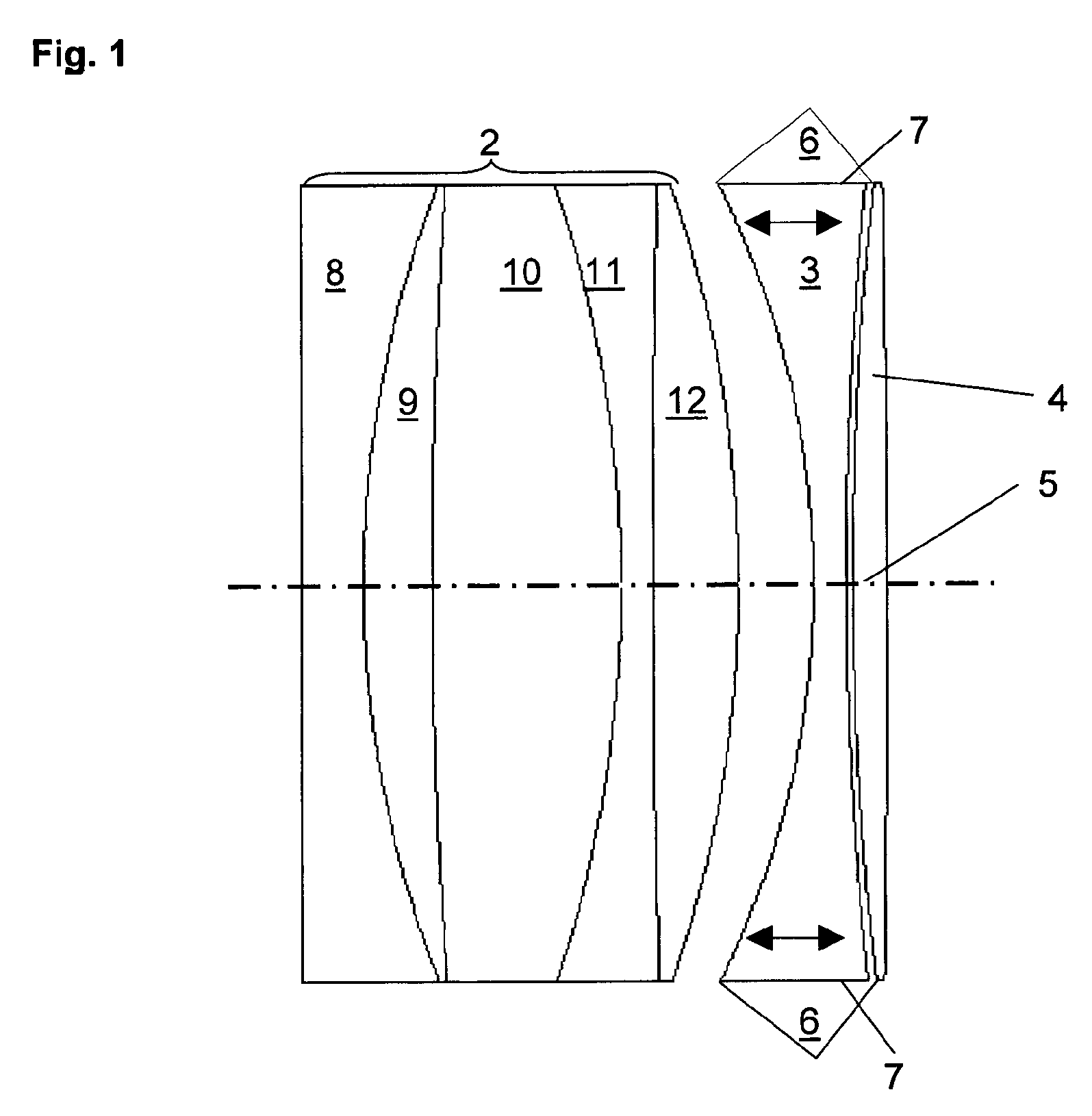
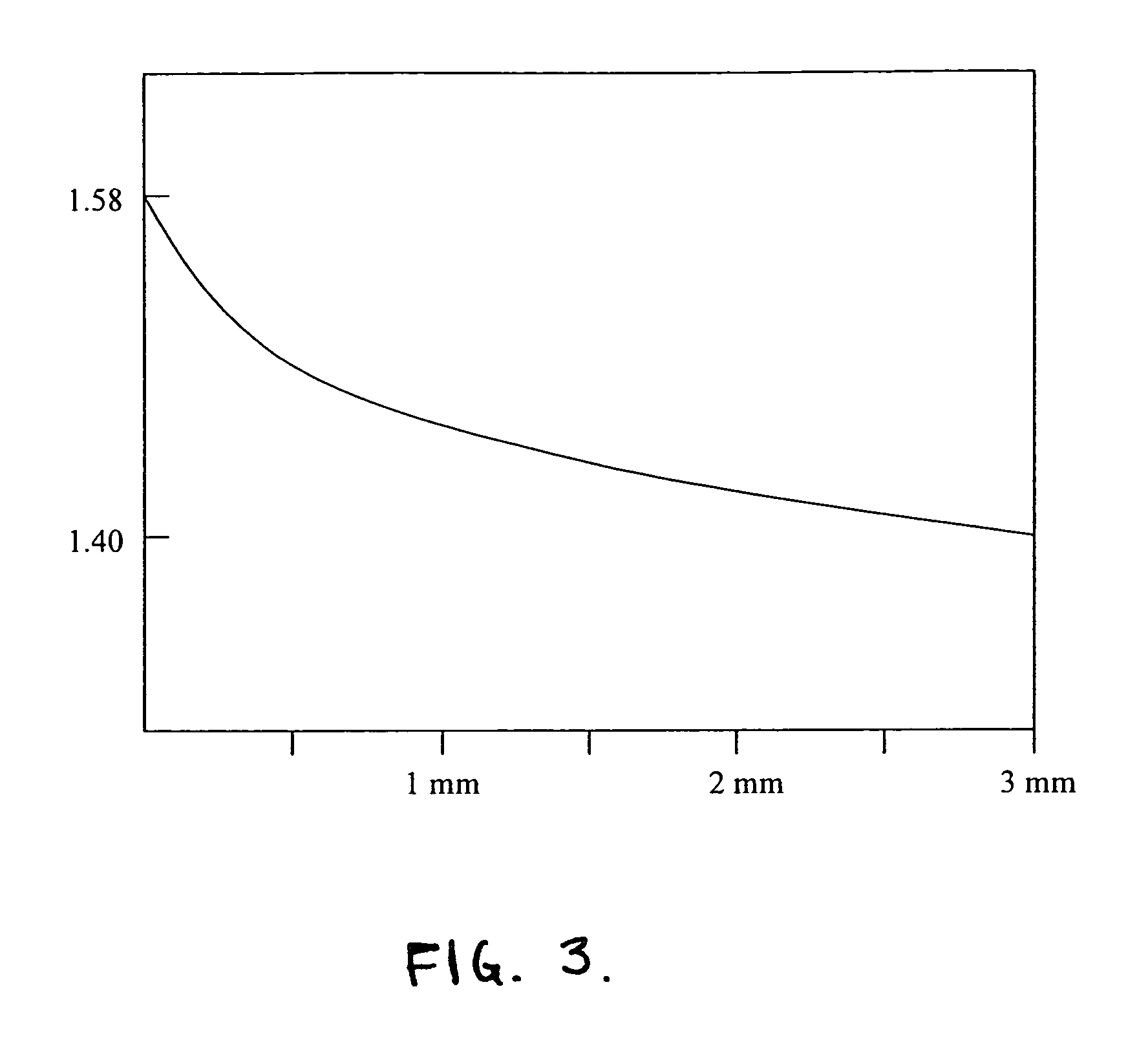
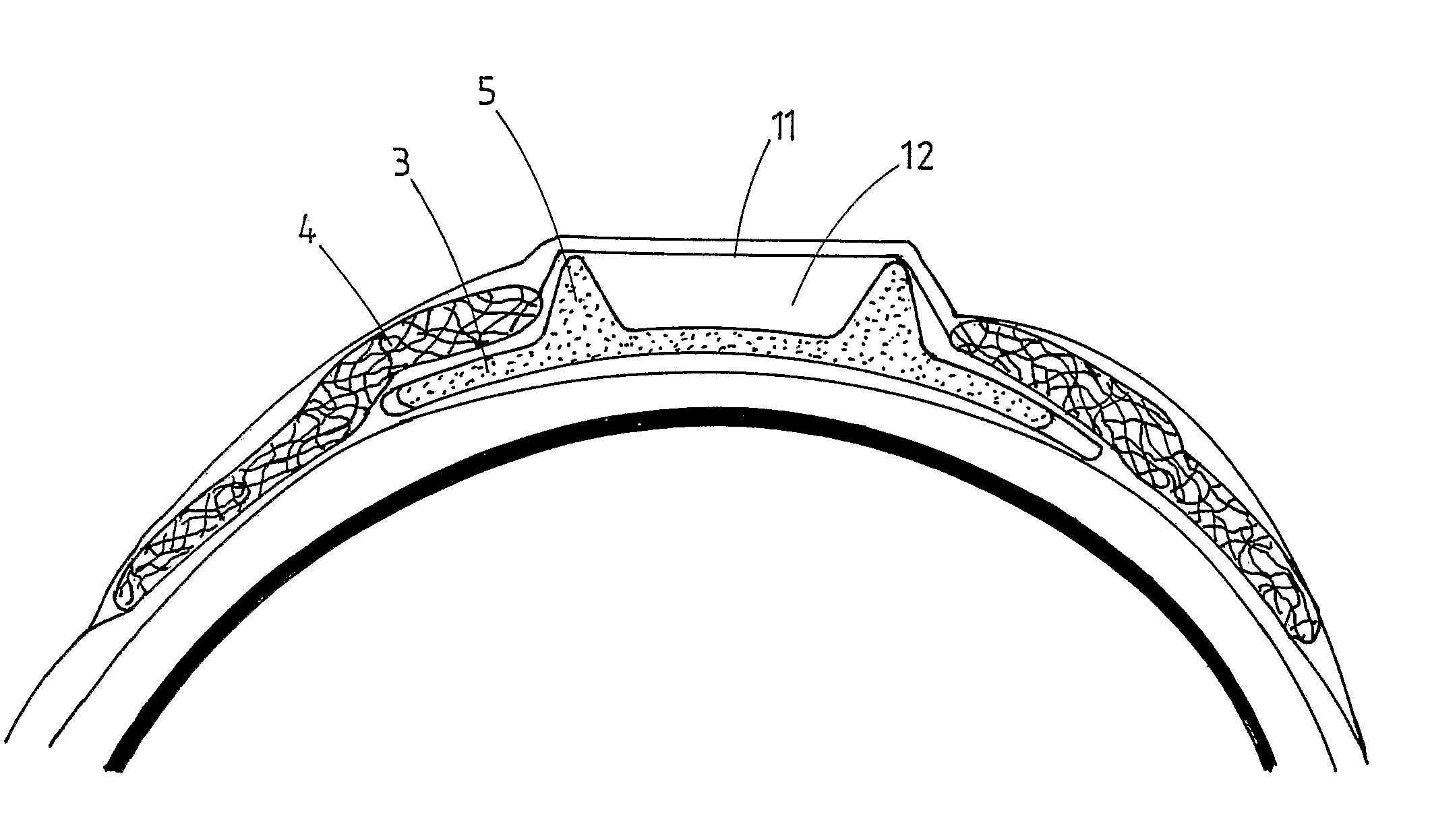
Wide-angle optical unit for ophthalmological implants
A wide-angle optical unit for ophthalmological implants in the eye includes a lens system arranged in a rotationally symmetrical manner about an optical axis and further includes at least two lenses that rest one on top of the other in a planar manner and which are made of materials having different optical refractive indices. In addition, the wide-angle optical unit includes an optical decoupling structure and a trailing lens arranged proximally with respect to the interior of the eye. The trailing lens is mounted around the optical decoupling structure.
Owner:KARLSRUHER INST FUR TECH
Infinite refractive index gradient (IRIG) polymers for ocular implant and contact lens applications
ActiveUS7857848B2Minimize of depthMinimize distortionOptical articlesEye diagnosticsOphthalmologyRefractive index
Infinite gradient refractive index ophthalmic devices and methods of making same. The method involves diffusing a monomer which polymerizes to a lower or higher corresponding opposite refractive index polymer into a lower or higher index polymer structure and polymerizing same to create the gradient structure. The resulting polymeric structure is used to manufacture ophthalmic devices, e.g., intraocular lenses.
Owner:KEY MEDICAL TECH
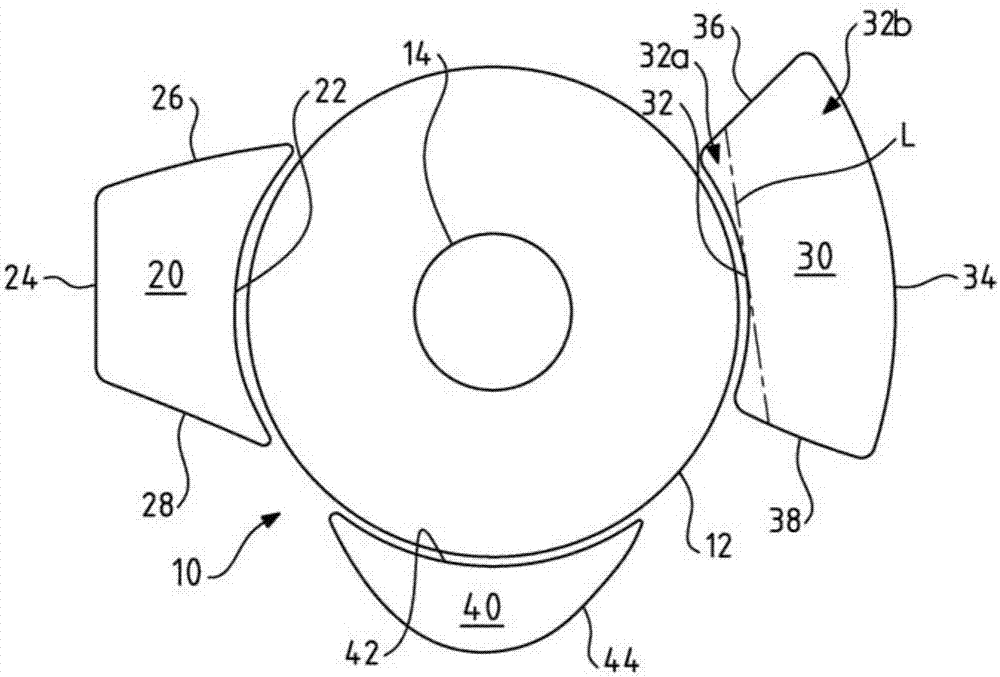
Ophthalmic Implant for Treating Glaucoma
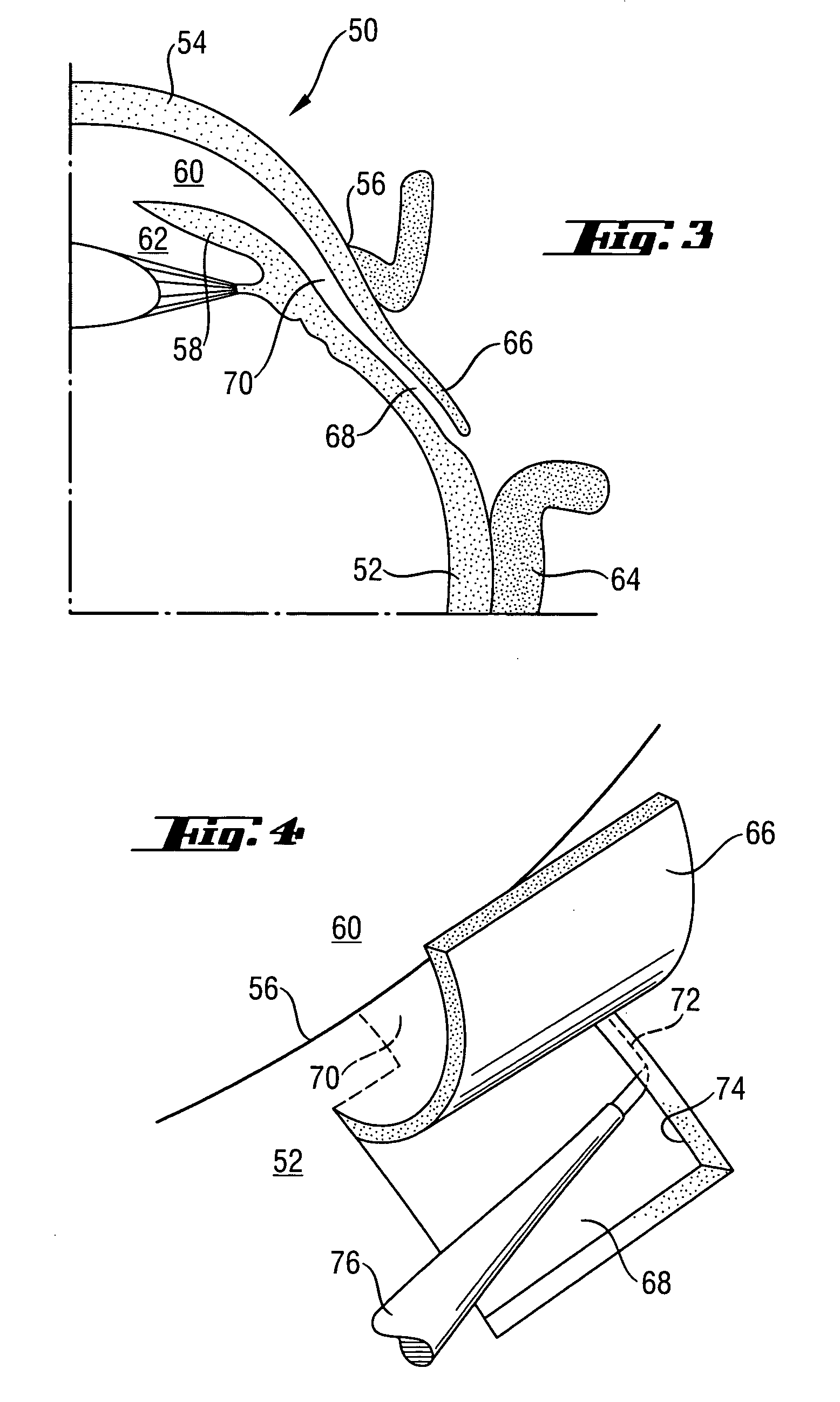
An ophthalmic implant for treating or alleviating the symptoms of glaucoma having a plate shaped to fit the surface of an eye. The plate has an inner ridge that defines a primary drainage region. Intraocular pressure is reduced by the transfer of aqueous fluid from the interior of the eyeball through a tube to the primary drainage region. To minimize post-operative hypotony, a secondary drainage region is provided on the plate outside the primary drainage region and defined either by the outer edge of the plate or an outer ridge having a lower profile than the inner ridge. When the pressure of fluid in the primary drainage region builds, fluid spills into the secondary drainage region. Fluid that is transferred to the plate is absorbed in surrounding tissues.
Owner:MOLTENO OPHTHALMIC
Ophthalmic implant for treatment of glaucoma
PendingUS20200121504A1Sufficient forceImprove permeabilityEye surgeryWound drainsOphthalmological implantSchlemm's canal
An implant is placed within Schlemm's canal of the eye and provides tension to the trabecular meshwork. The tension is continuous and increases the aqueous outflow without the necessity of administering cholinergic drugs to treat glaucoma.
Owner:NOVA EYE INC
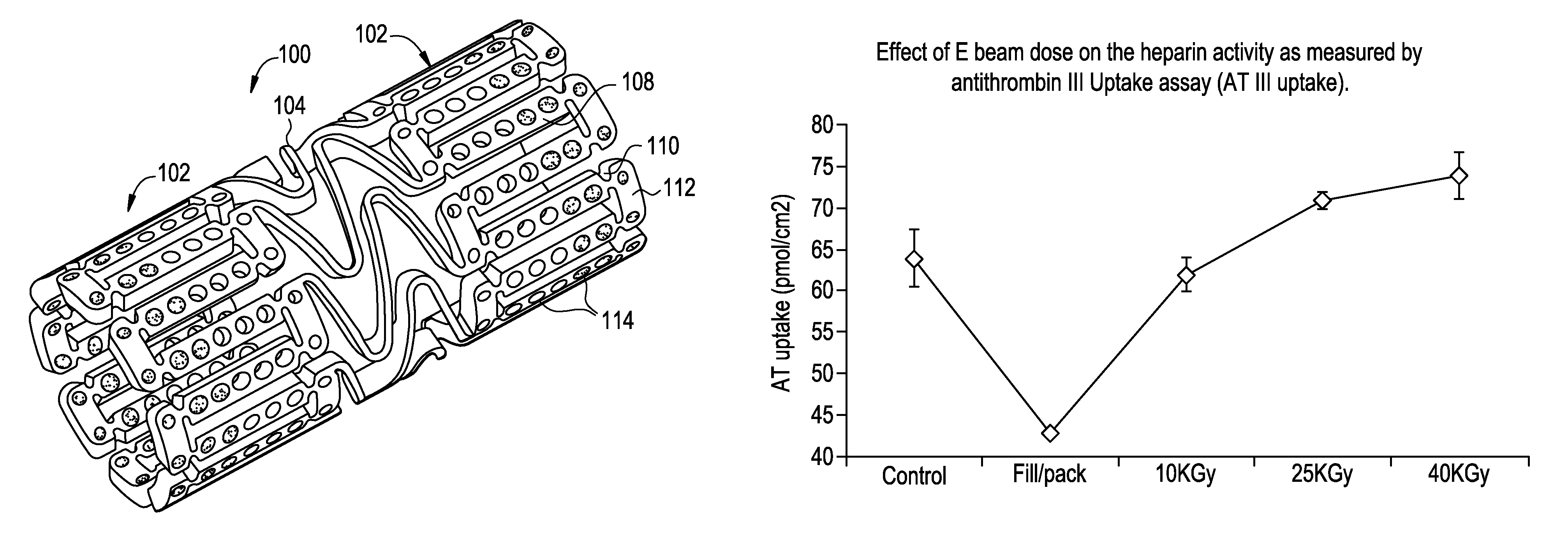
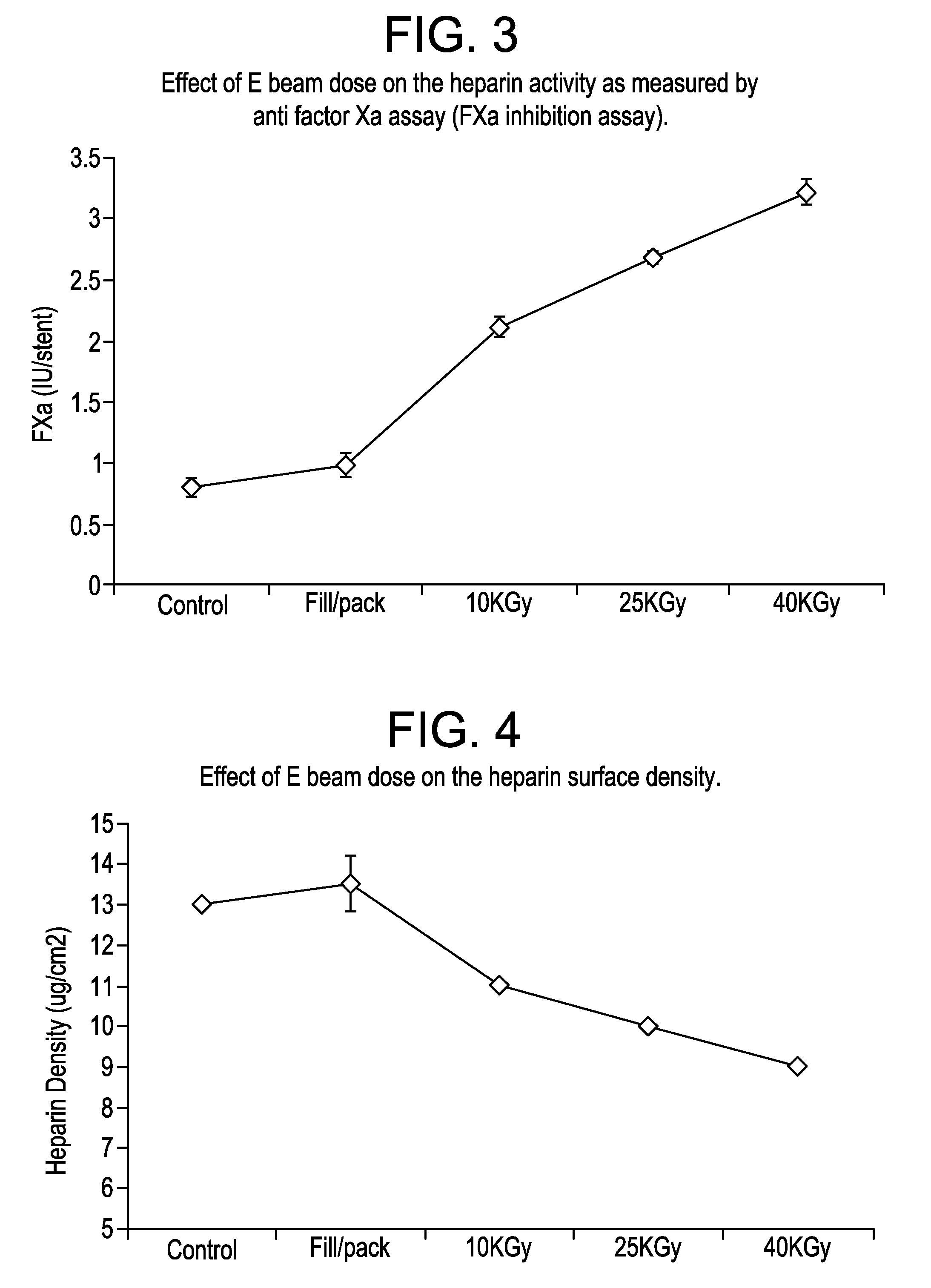
E beam sterilization of medical devices comprising bioactive coating
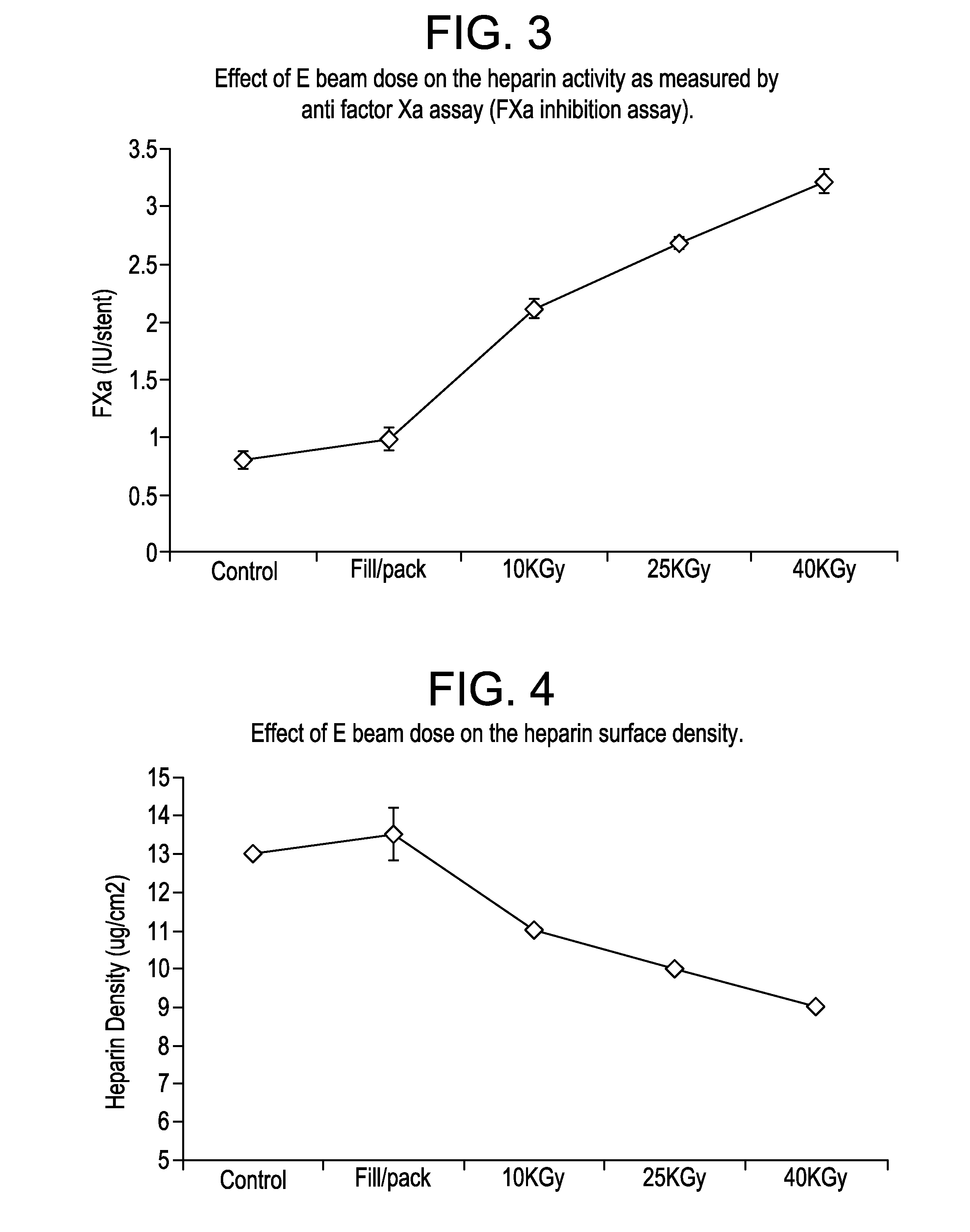
ActiveUS20110113728A1Extended shelf lifeMaintain biological activityPackage sterilisationPackaging by pressurising/gasifyingHeparin coatingOphthalmological implant
The invention provides a method for single-step terminal sterilization process for bio-active heparin coatings on materials and biomaterials containing heparin used in medical devices, such as catheters, tissue engineering scaffolds, or drug delivery carrier materials. This may include any medical device or implantable that could benefit from improved antithrombotic and biocompatible heparin surfaces. Other relevant device examples may include heparin or a heparin derivative coated stents to reduce clotting and restenosis, dental or ophthalmological implants. These materials may comprise additional polymeric compositions such as polyethyleneimine, dextran sulfate or their modified forms. These polymers together with heparin coatings may be applied to other substrate of medical devices such as metal, ceramics or biologically derived materials.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
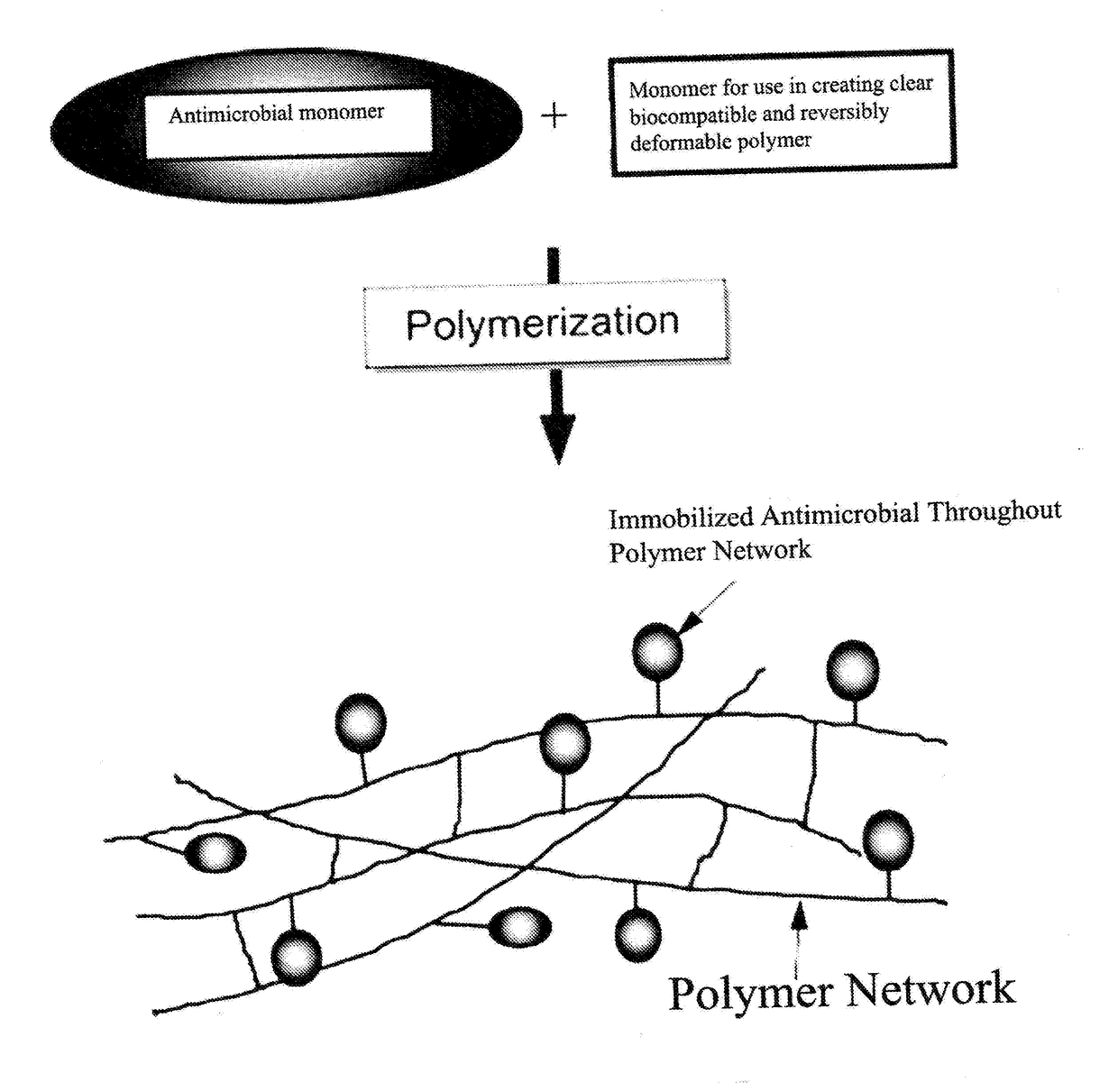
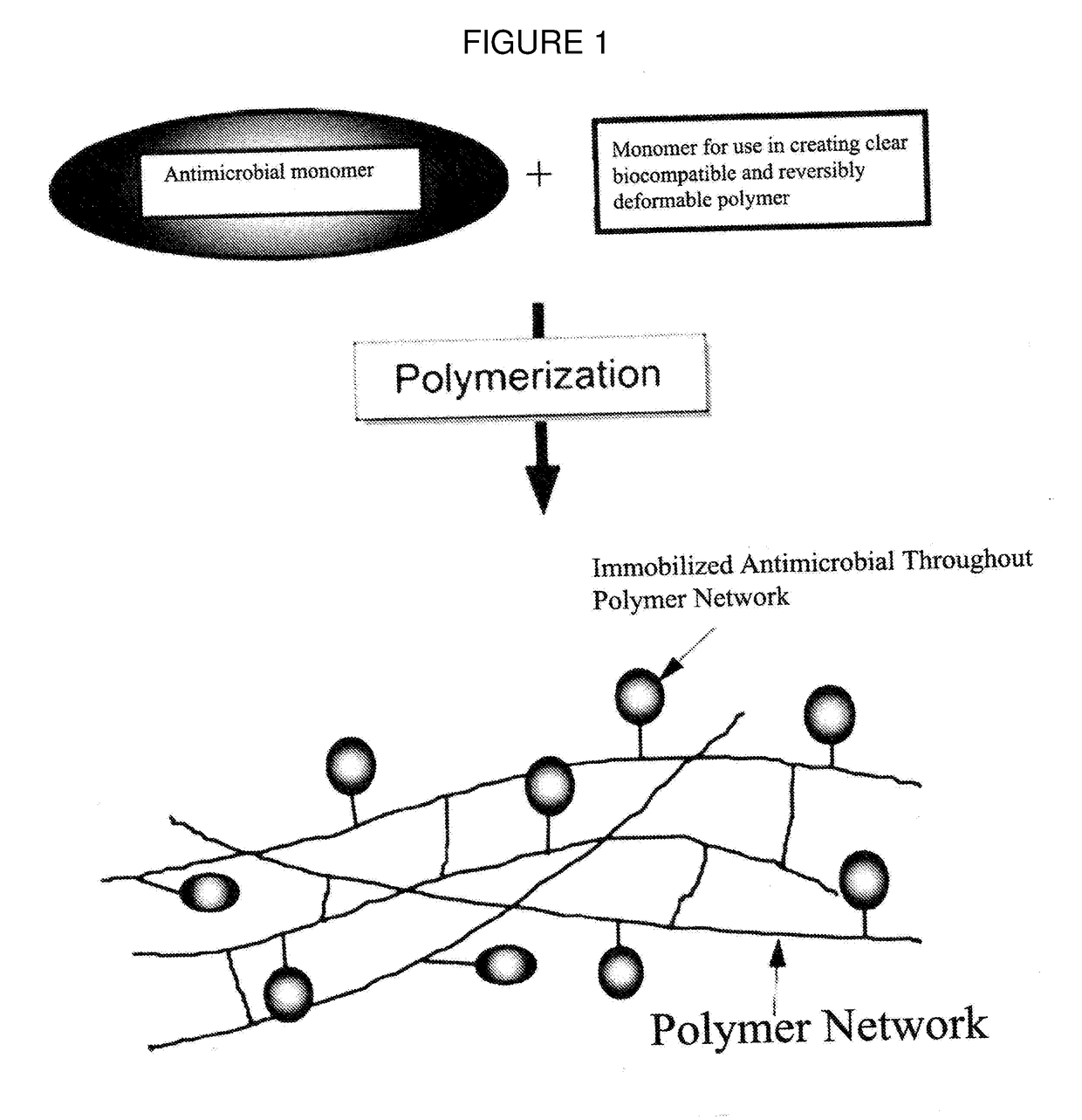
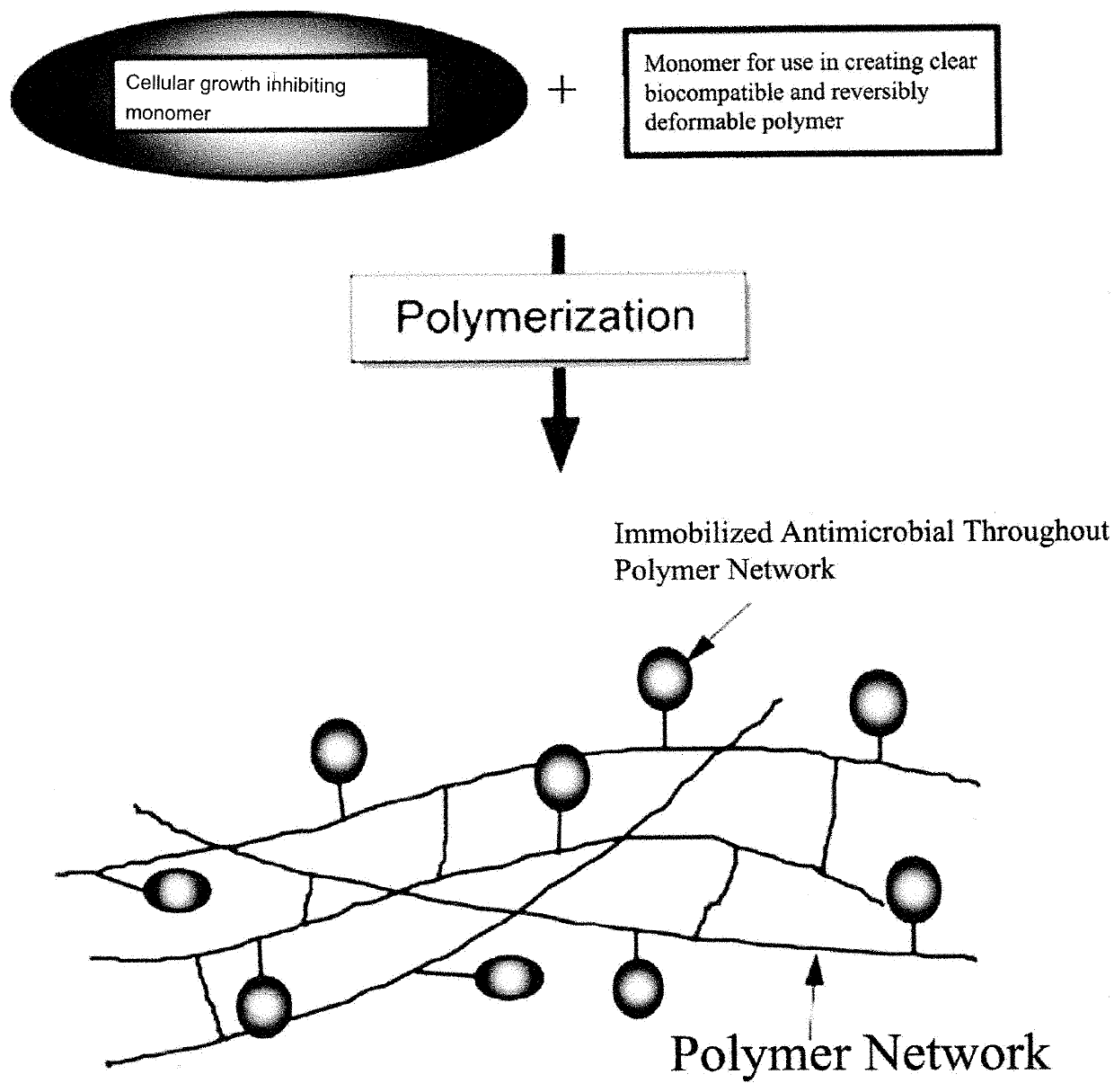
Antimicrobial Polymer for Use in Ophthalmic implants
ActiveUS20180064643A1Reduce the risk of infectionFunction increaseAntibacterial agentsEye implantsOphthalmological implantOphthalmology
An antimicrobial polymer for use in an ophthalmic implant, includes at least one antimicrobial monomer; and at least one other monomer selected from an acrylic, silicone, vinyl and collagen monomer.
Owner:KERAMED
Ophthalmic implant for treating glaucoma
ActiveUS7776002B2Eye surgeryOther blood circulation devicesOphthalmological implantIntraocular pressure
An ophthalmic implant for treating or alleviating the symptoms of glaucoma having a plate shaped to fit the surface of an eye. The plate has an inner ridge that defines a primary drainage region. Intraocular pressure is reduced by the transfer of aqueous fluid from the interior of the eyeball through a tube to the primary drainage region. To minimize post-operative hypotony, a secondary drainage region is provided on the plate outside the primary drainage region and defined either by the outer edge of the plate or an outer ridge having a lower profile than the inner ridge. When the pressure of fluid in the primary drainage region builds, fluid spills into the secondary drainage region. Fluid that is transferred to the plate is absorbed in surrounding tissues.
Owner:MOLTENO OPHTHALMIC
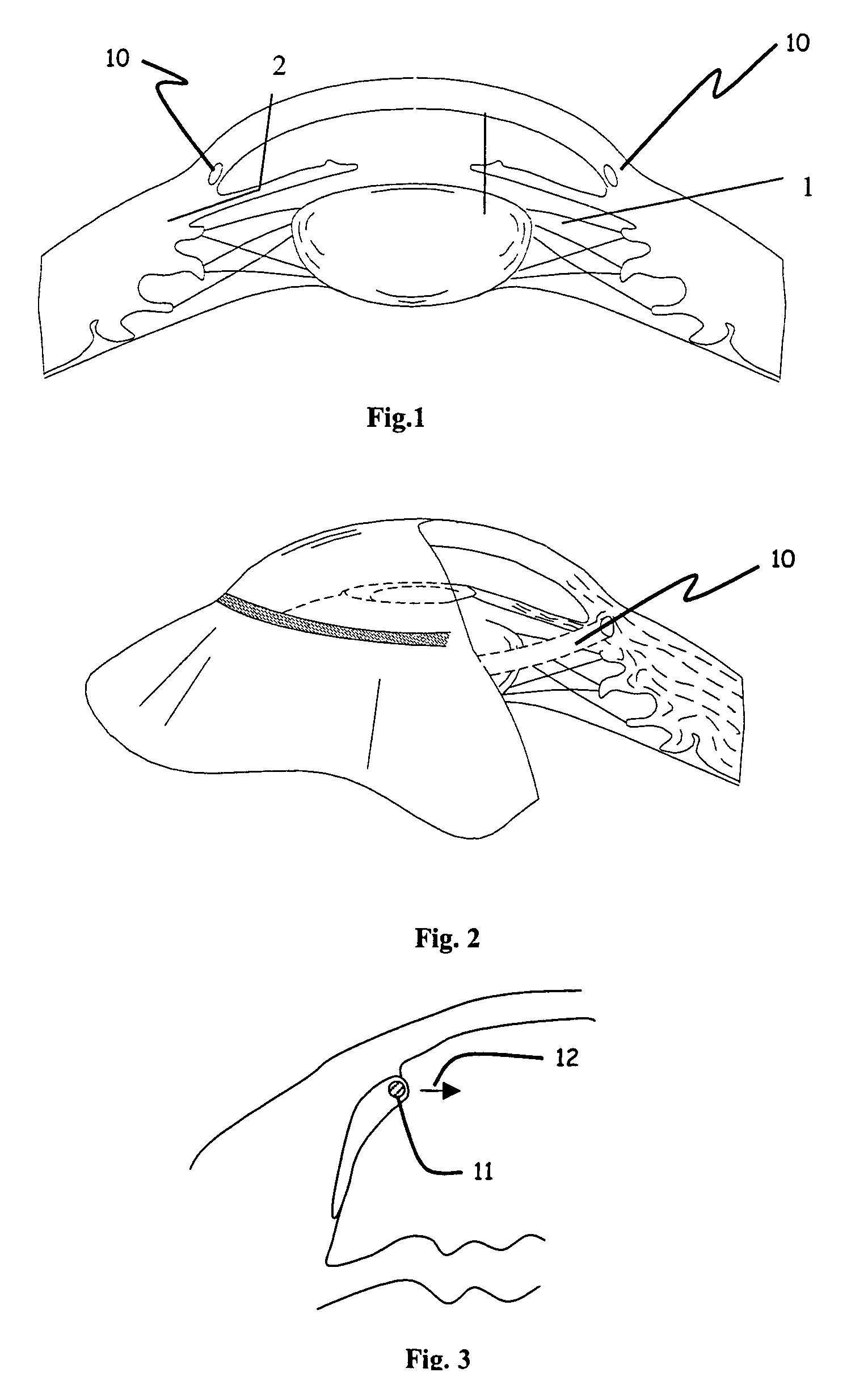
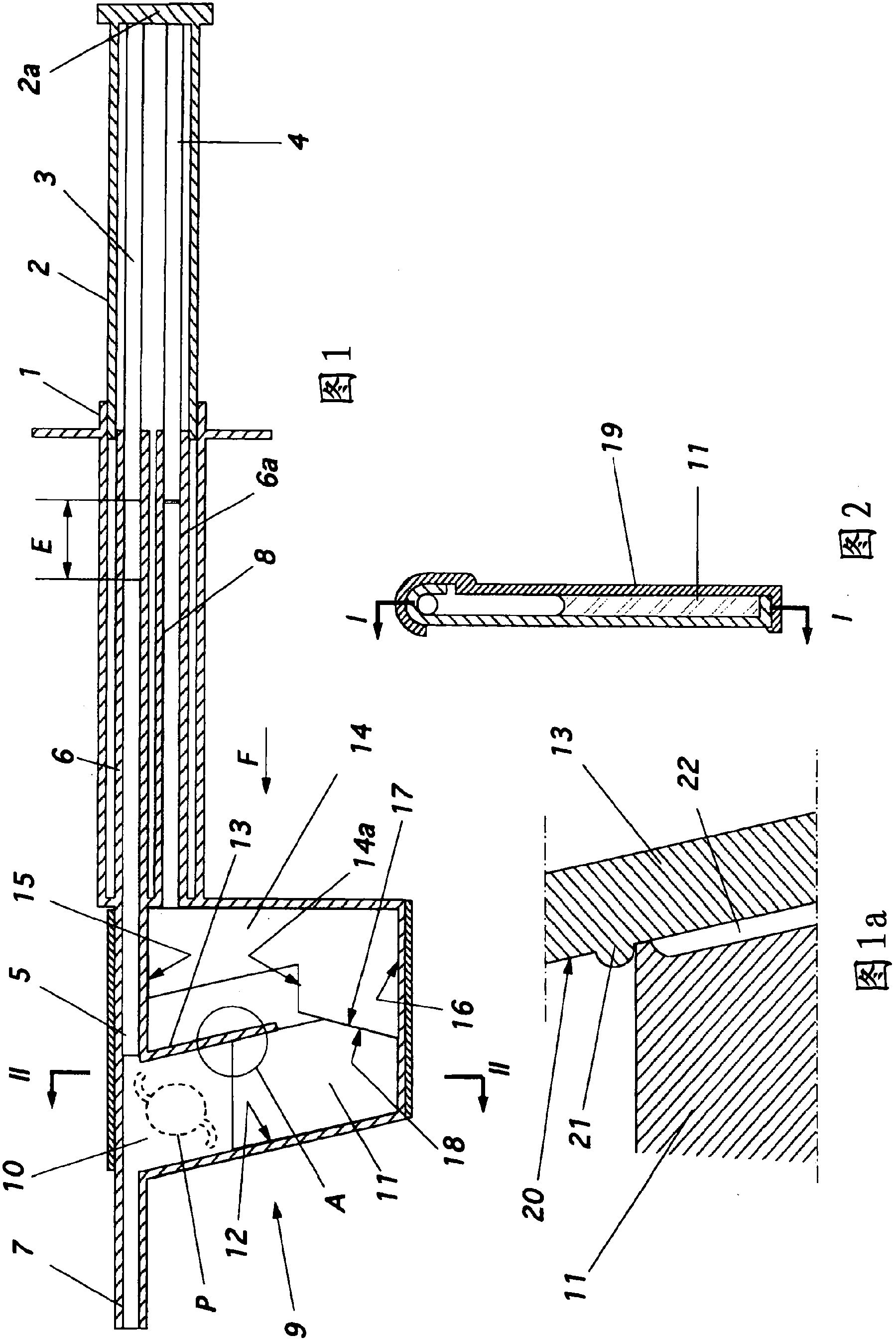
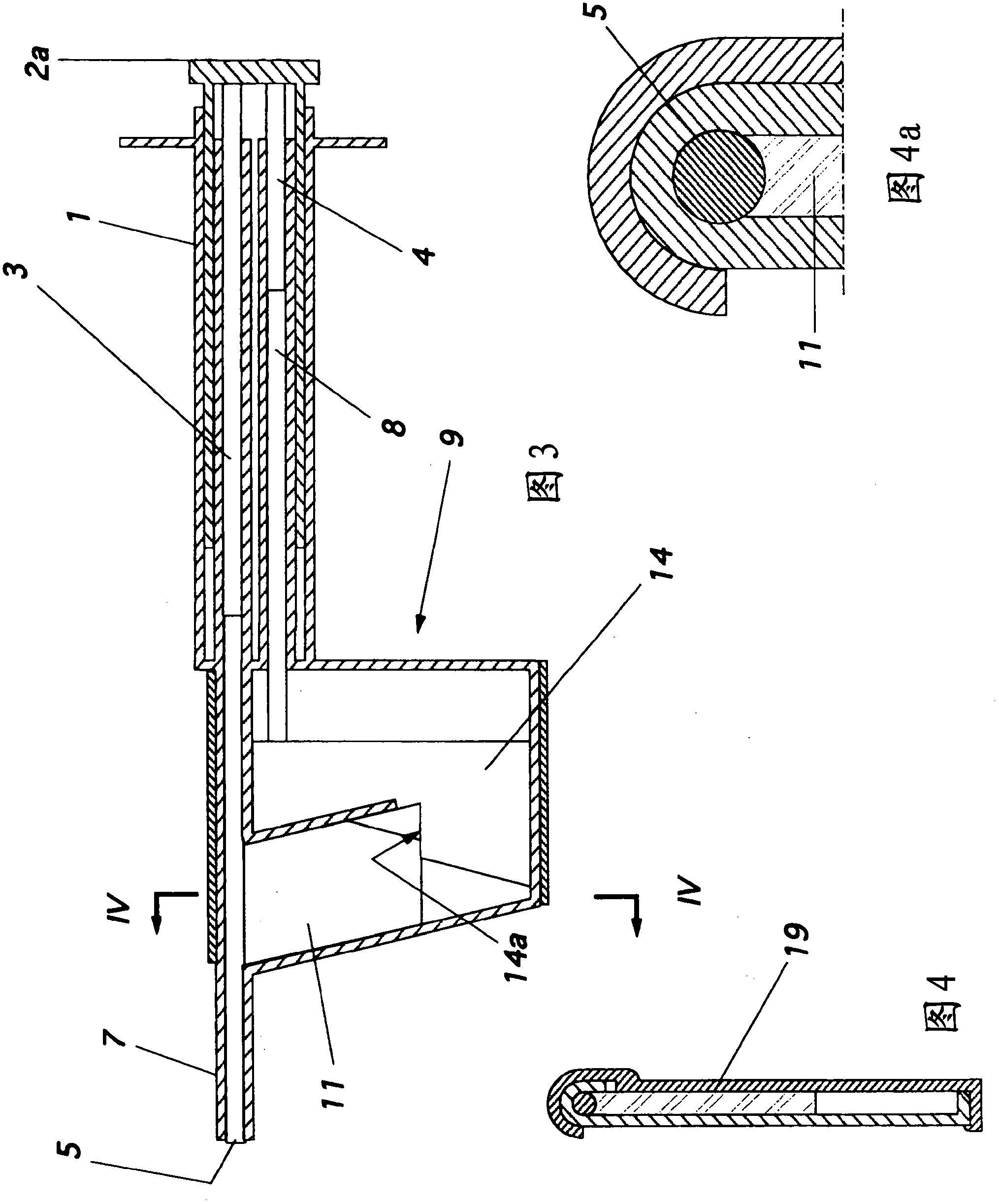
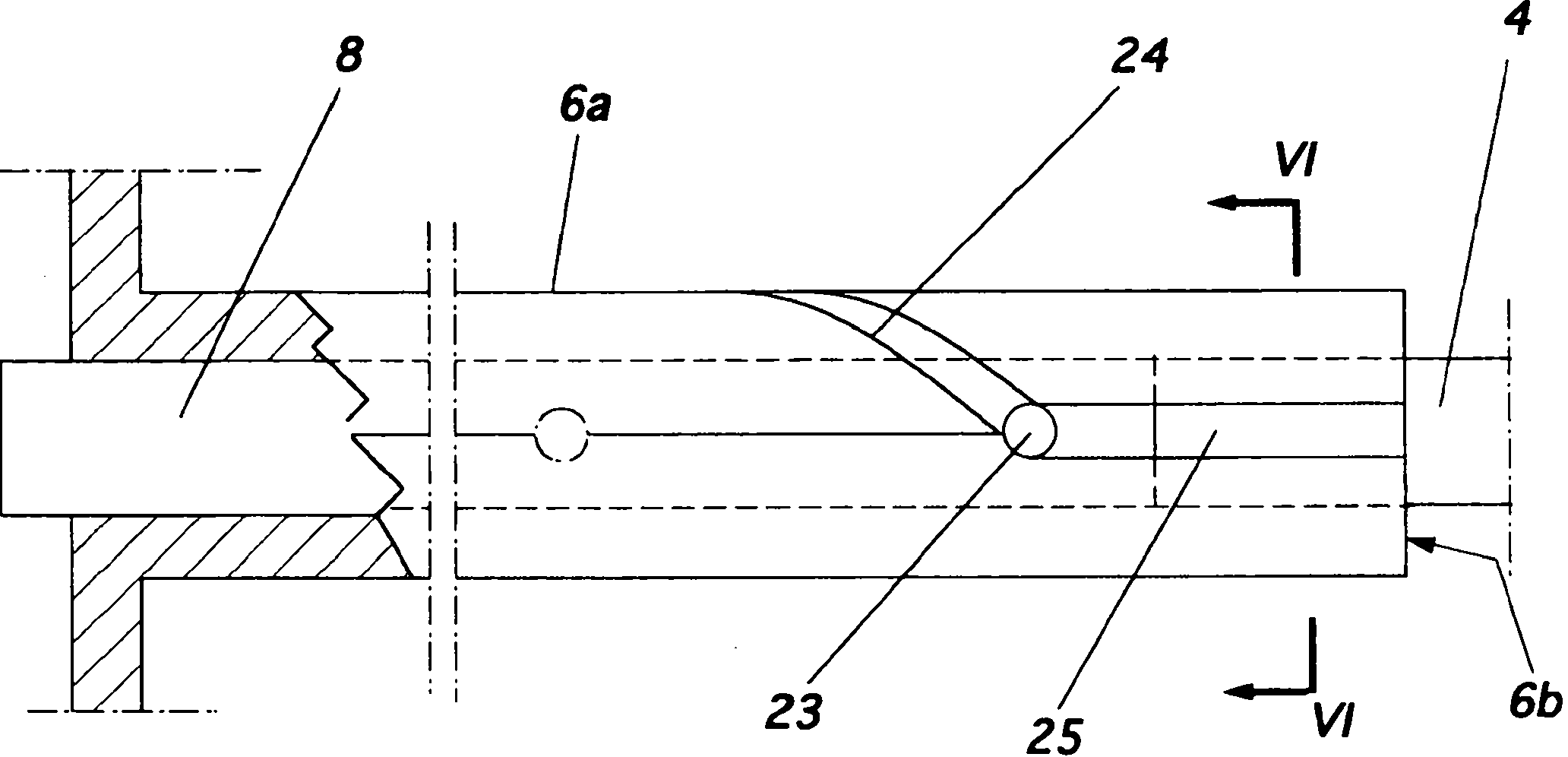
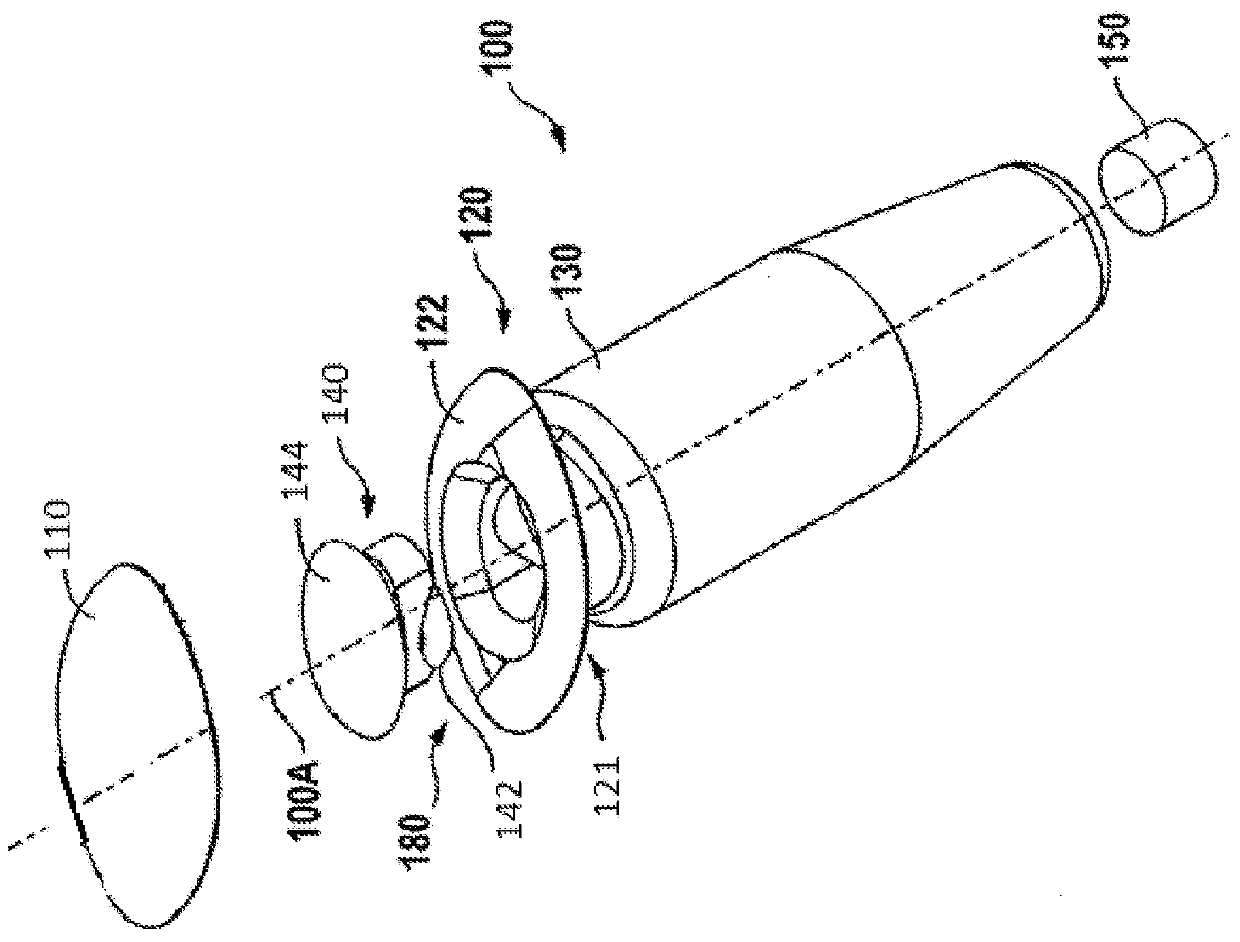
Injector for a flexible ophthalmologic implant
The invention relates to an injector characterised in that said injector comprises a general tappet (2) having two rods (3, 4), one of which can engage with an ejection tappet (5) while the other can engage, via the middle section of a folding tappet (8), with a mechanism enabling the implant (P) to be folded and inserted in a hollow needle (7), a means being provided for inhibiting, when desirable, the action of either one of the rods of the tappet.
Owner:萨迪克・穆哈贝丁
Ophthalmological implant and method for the production of same
Owner:CARL ZEISS MEDITEC AG
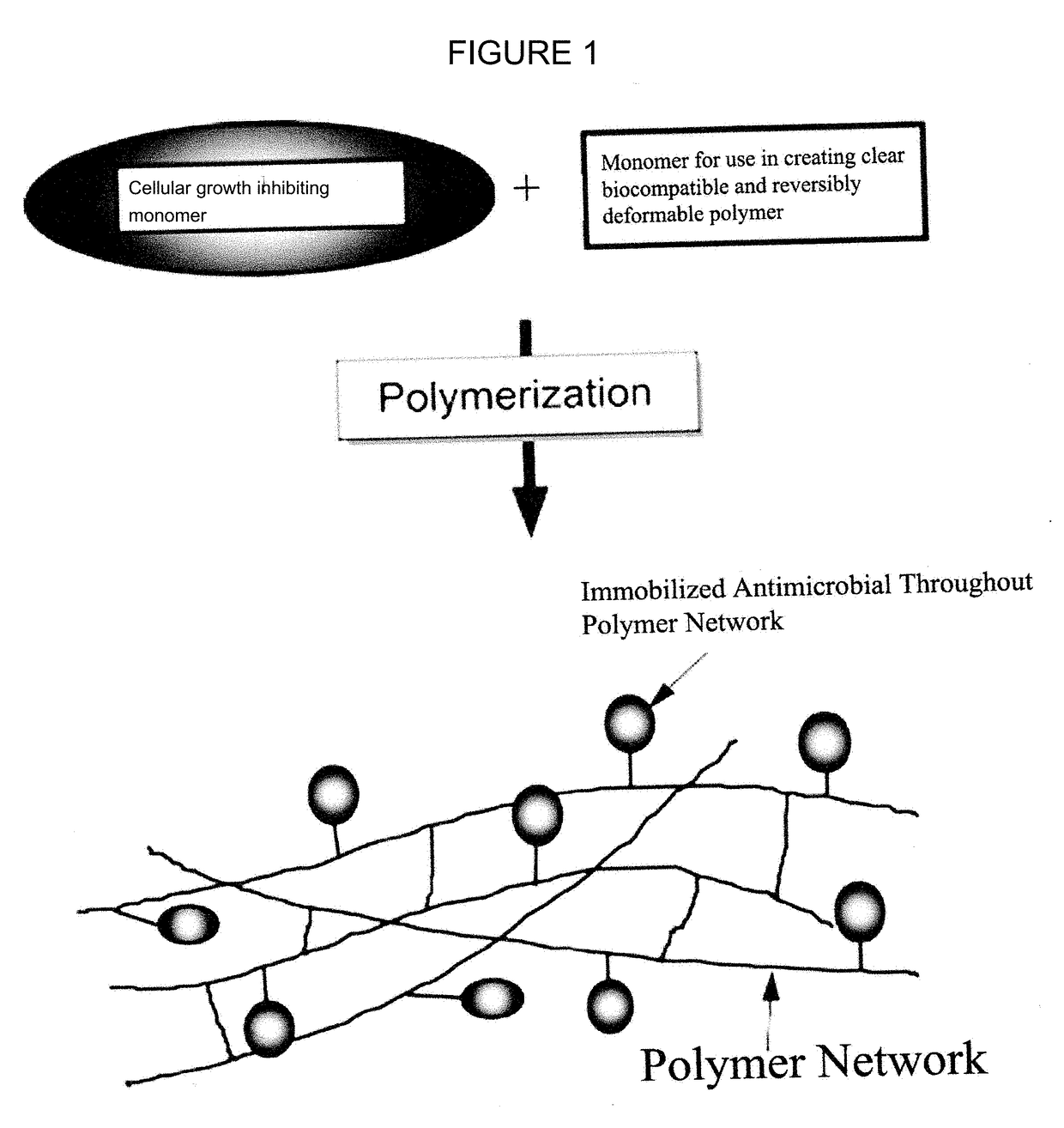
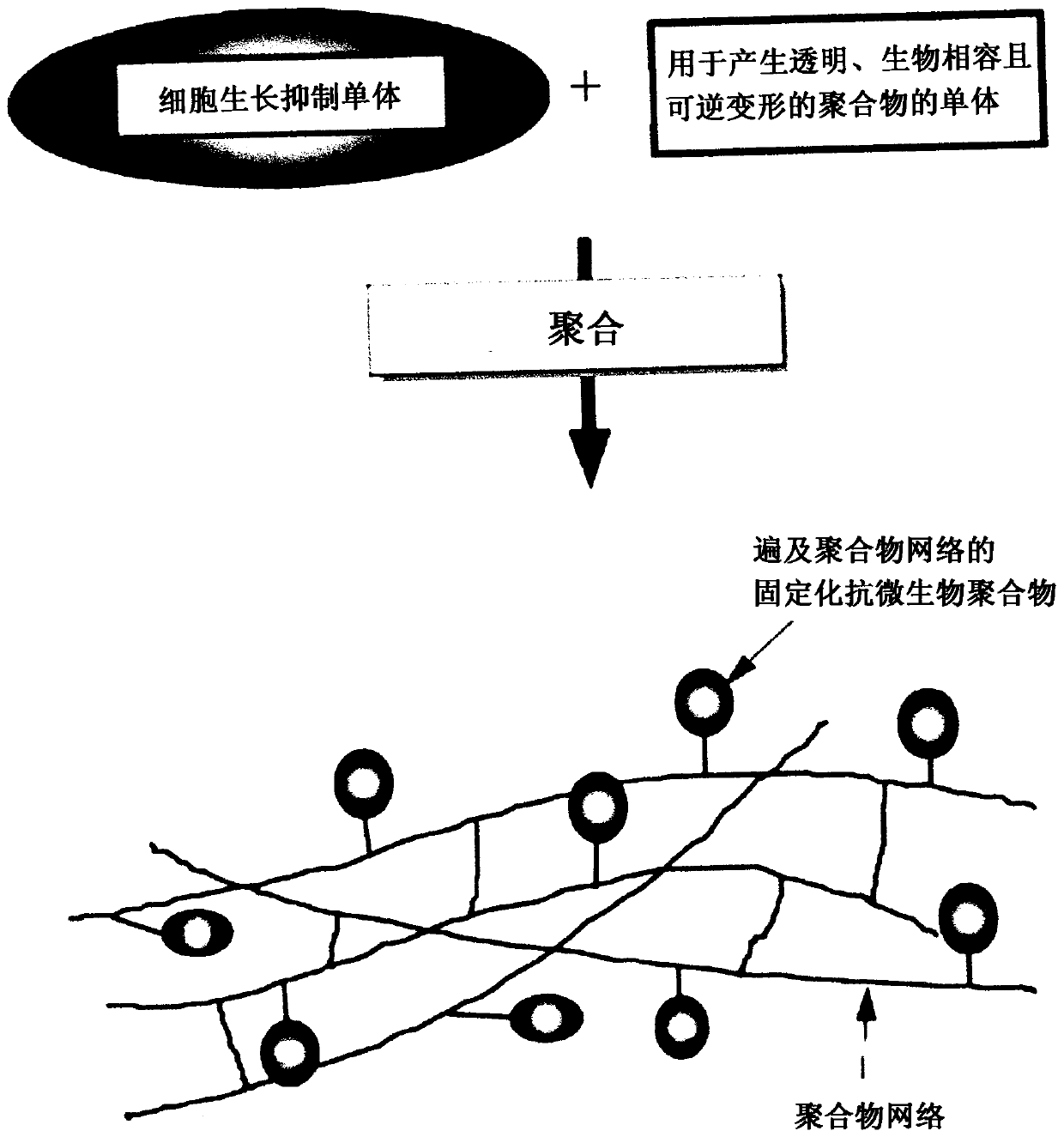
Cell growth inhibiting copolymer for use in ophthalmic implants
ActiveUS20180361018A1Function increaseInhibit cell growthProsthesisOphthalmological implantCell growth
A cell growth inhibiting polymer for use in an ophthalmic implant includes at least one cell growth inhibiting monomer; and at least one other monomer selected from an acrylic monomer, a hydrophobic acrylic monomer, a hydrophilic acrylic monomer, a silicone monomer, a vinyl monomer and / or a collagen monomer.
Owner:KERAMED
Hydrogel copolymers for biomedical devices
Hydrogel copolymers which are hydrated polymerization products of monomeric mixtures comprising polysiloxane prepolymers and hydrophilic comonomers are useful for forming biomedical devices, particularly ophthalmic devices including contact lenses, intraocular lenses and ophthalmic implants. The copolymers have a desirable combination of oxygen permeability, tensile modulus, and water content, especially for soft contact lenses.
Owner:BAUSCH & LOMB INC
Composition for ophthalmological products
ActiveUS20200332041A1Advantageous unfolding timeOptical elementsOphthalmological implantOphthalmology
Owner:JOHNSON & JOHNSON SURGICAL VISION INC
Ophthalmic implants for delivery of therapeutic substances
Owner:FORSIGHT VISION5 INC
Cell growth inhibiting copolymer for use in ophthalmic implants
ActiveUS11185609B2Function increaseInhibit cell growthProsthesisOphthalmological implantOphthalmology
A cell growth inhibiting polymer for use in an ophthalmic implant includes at least one cell growth inhibiting monomer; and at least one other monomer selected from an acrylic monomer, a hydrophobic acrylic monomer, a hydrophilic acrylic monomer, a silicone monomer, a vinyl monomer and / or a collagen monomer.
Owner:KERAMED
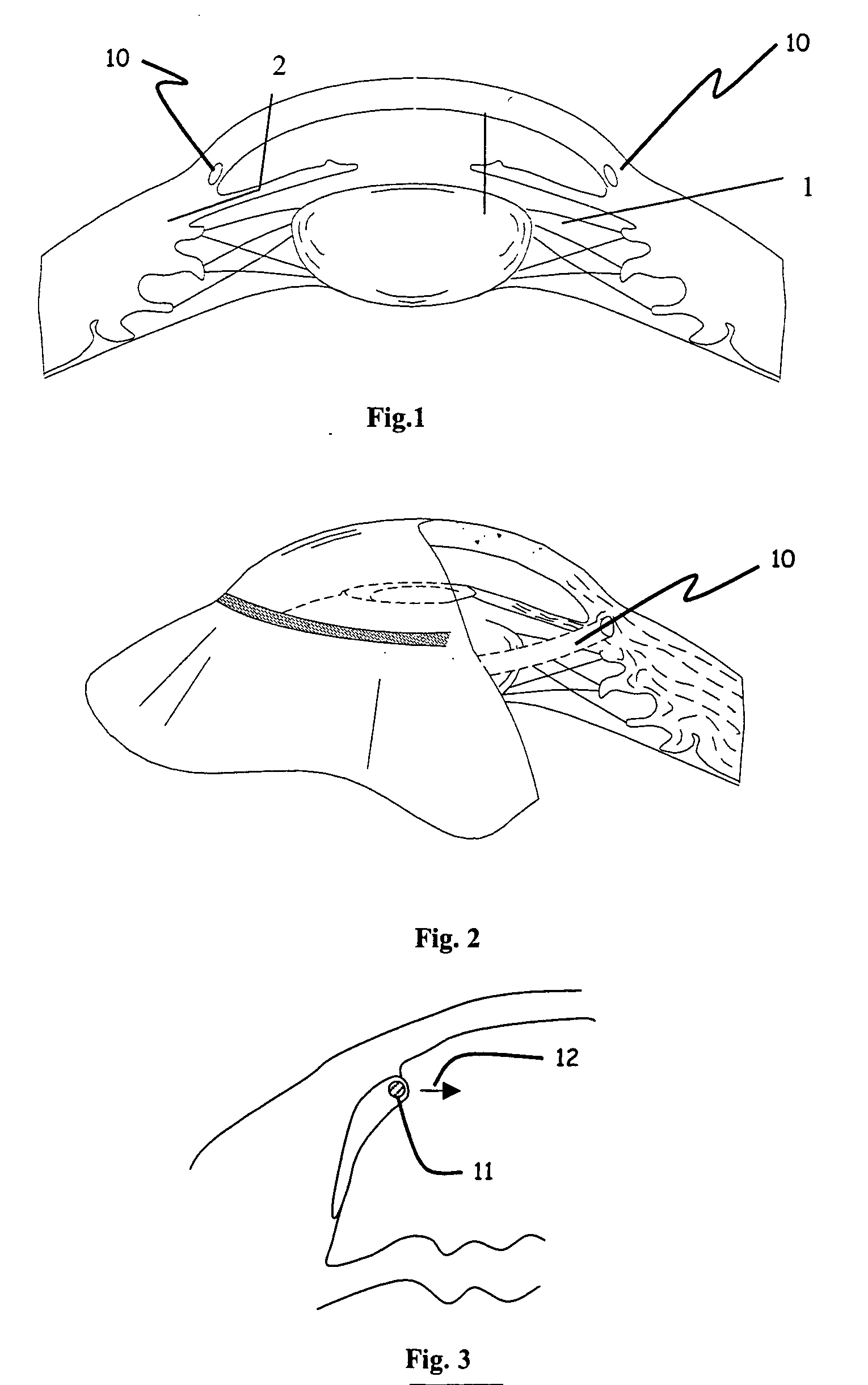
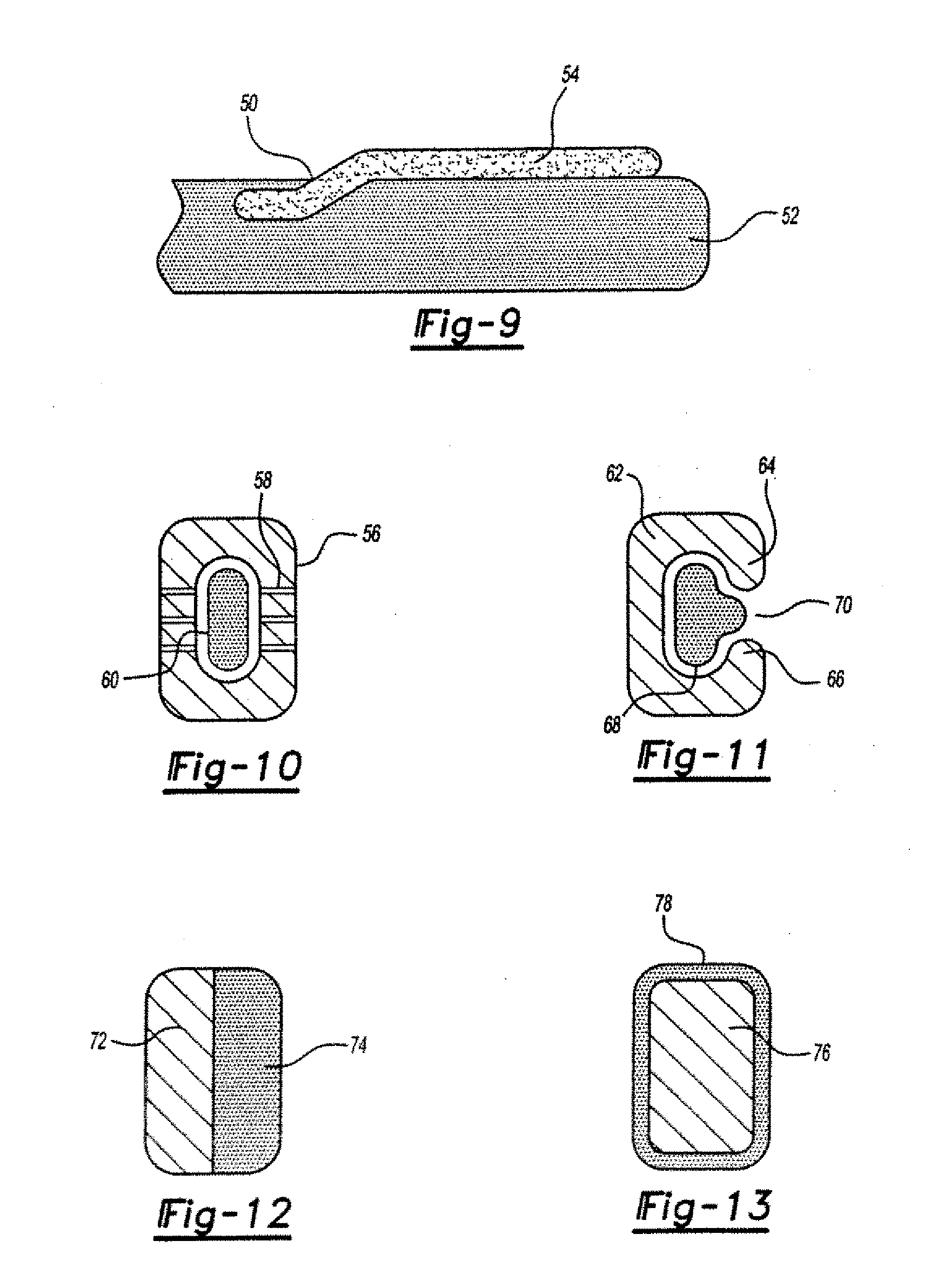
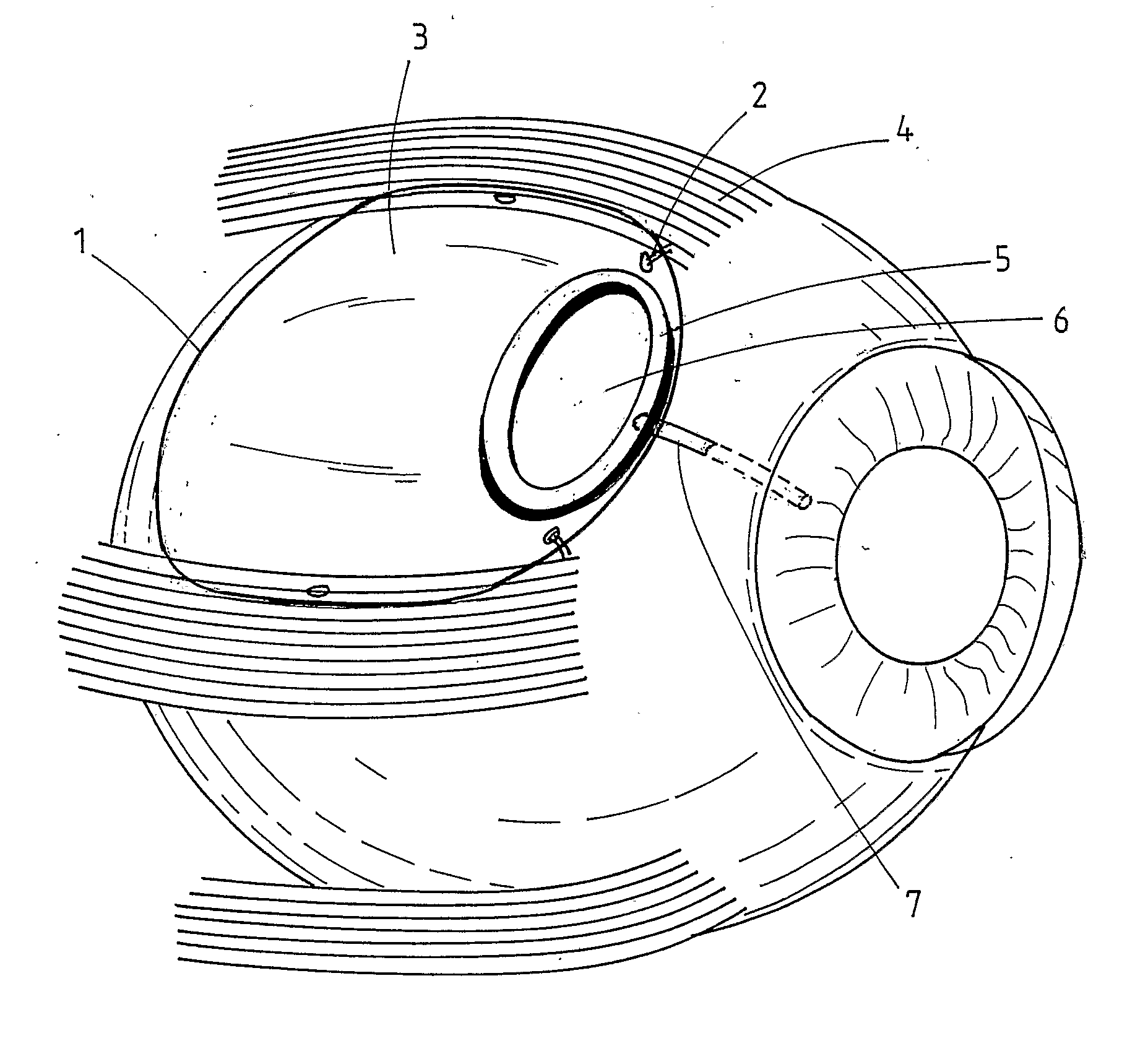
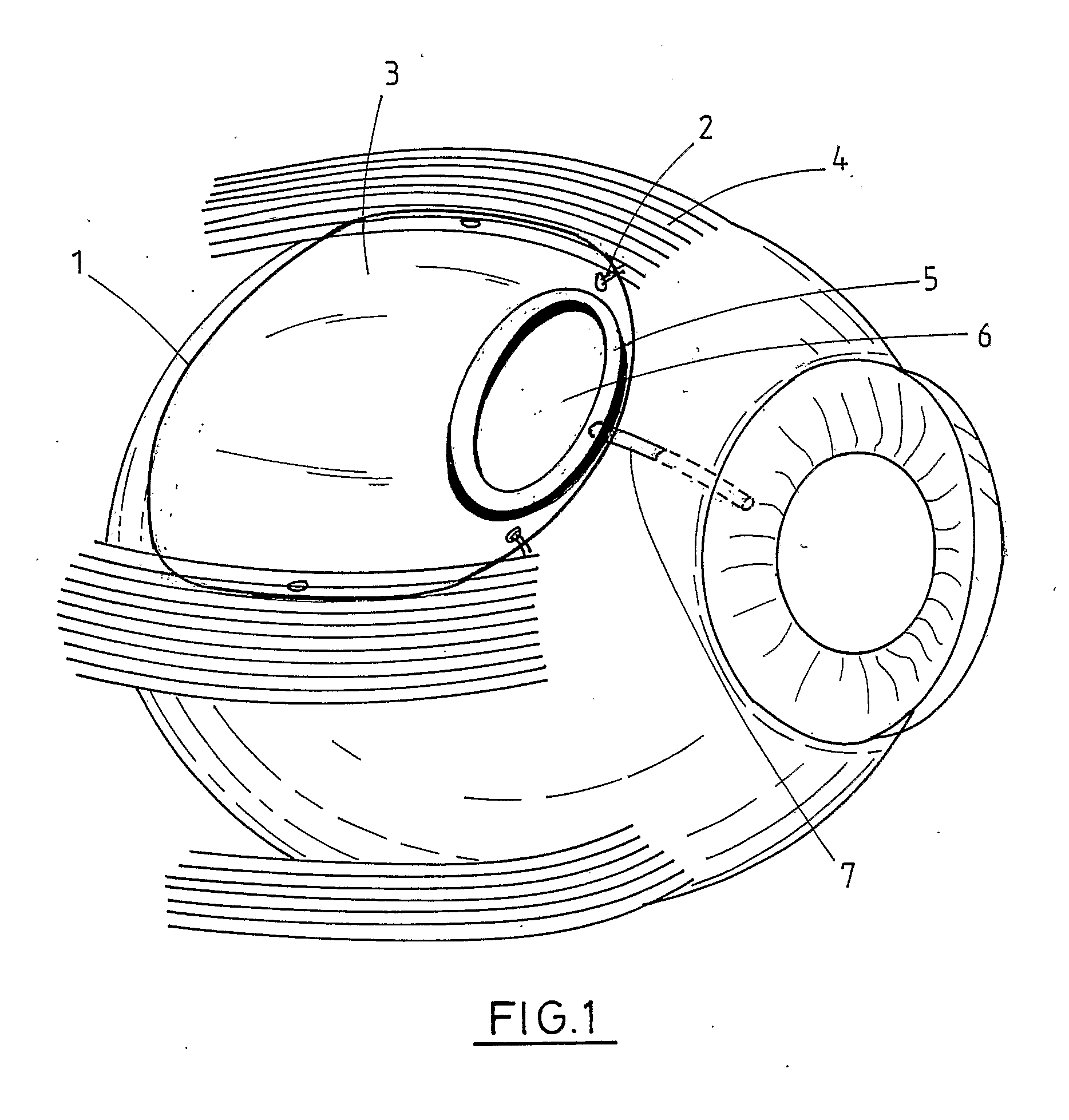
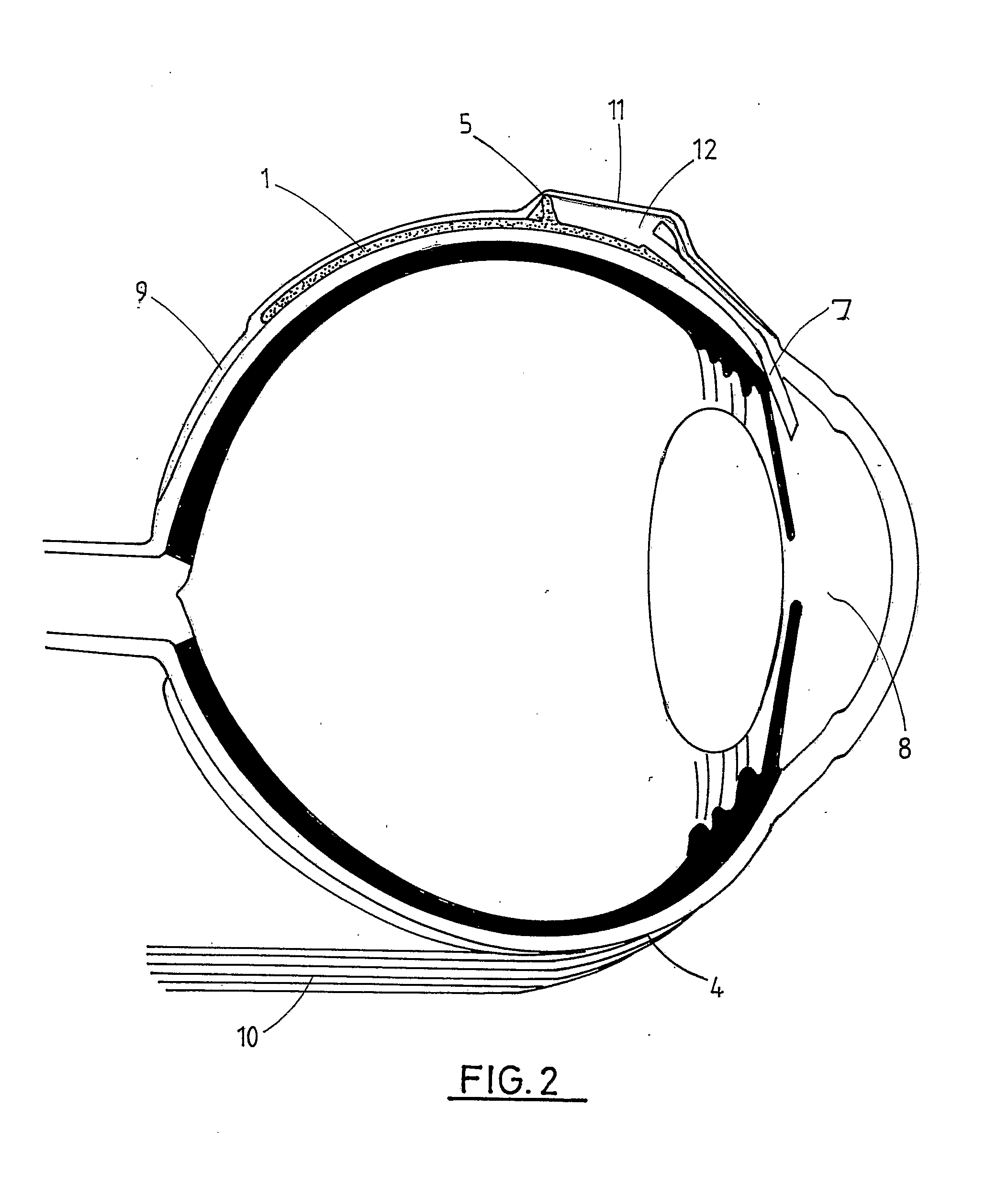
Interpositional ophthalmological implant
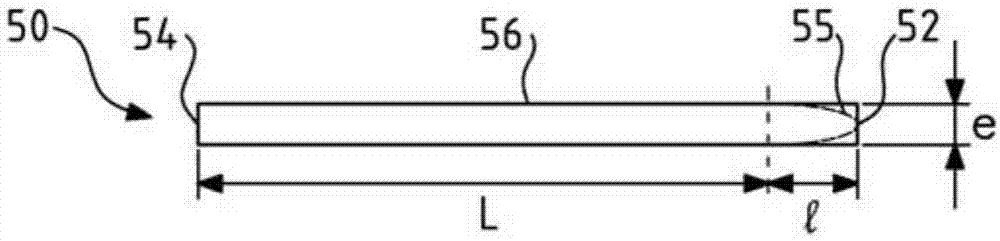
The invention relates to a permanent interpositional ophthalmological implant between the sclera and the uveal tissue. The implant comprises a thin uvea-compatible body (50) having a thickness (e) which is at least less than 10 times the smallest of the two other dimensions of the body, the body of the implant comprising two opposing edges separated from one another along one of the two dimensionsperpendicular to the thickness, and one of the edges, known as the front edge (52), being curved in a concave manner away from body in a plane perpendicular to the thickness.
Owner:希里亚泰克公司
E beam sterilization of medical devices comprising bioactive coating
ActiveUS8887477B2Extended shelf lifeMaintain biological activityPackage sterilisationLavatory sanitoryHeparin coatingOphthalmological implant
The invention provides a method for single-step terminal sterilization process for bio-active heparin coatings on materials and biomaterials containing heparin used in medical devices, such as catheters, tissue engineering scaffolds, or drug delivery carrier materials. This may include any medical device or implantable that could benefit from improved antithrombotic and biocompatible heparin surfaces. Other relevant device examples may include heparin or a heparin derivative coated stents to reduce clotting and restenosis, dental or ophthalmological implants. These materials may comprise additional polymeric compositions such as polyethyleneimine, dextran sulfate or their modified forms. These polymers together with heparin coatings may be applied to other substrate of medical devices such as metal, ceramics or biologically derived materials.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Cell growth inhibiting copolymer for use in ophthalmic implants
PendingCN110753859AGrowth inhibitionFunction increasePharmaceutical non-active ingredientsProsthesisOphthalmological implantOphthalmology
A cell growth inhibiting polymer for use in an ophthalmic implant includes at least one cell growth inhibiting monomer; and at least one other monomer selected from an acrylic monomer, a hydrophobic acrylic monomer, a hydrophilic acrylic monomer, a silicone monomer, a vinyl monomer and / or a collagen monomer.
Owner:伊齐耶·舒埃
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com