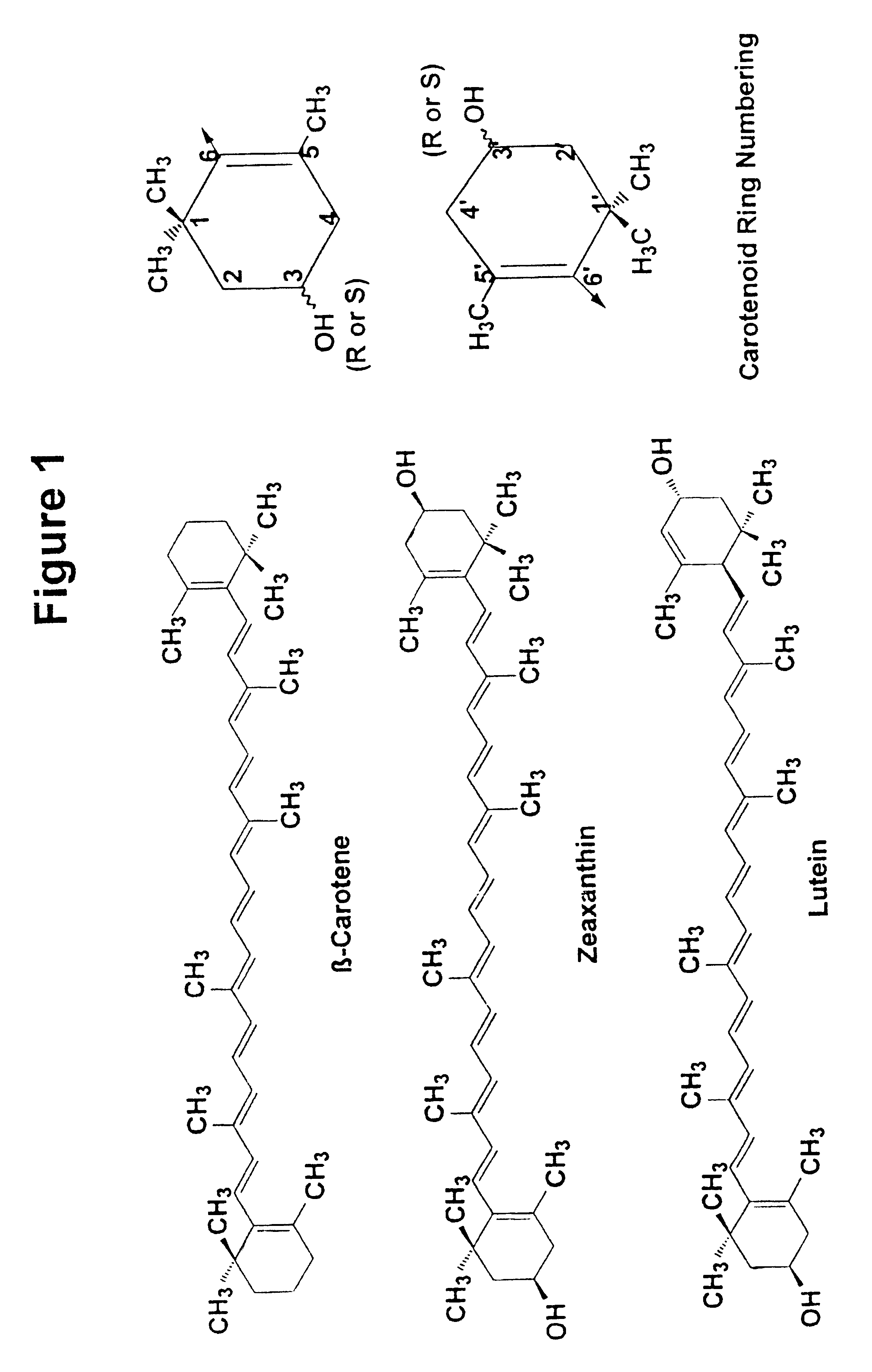
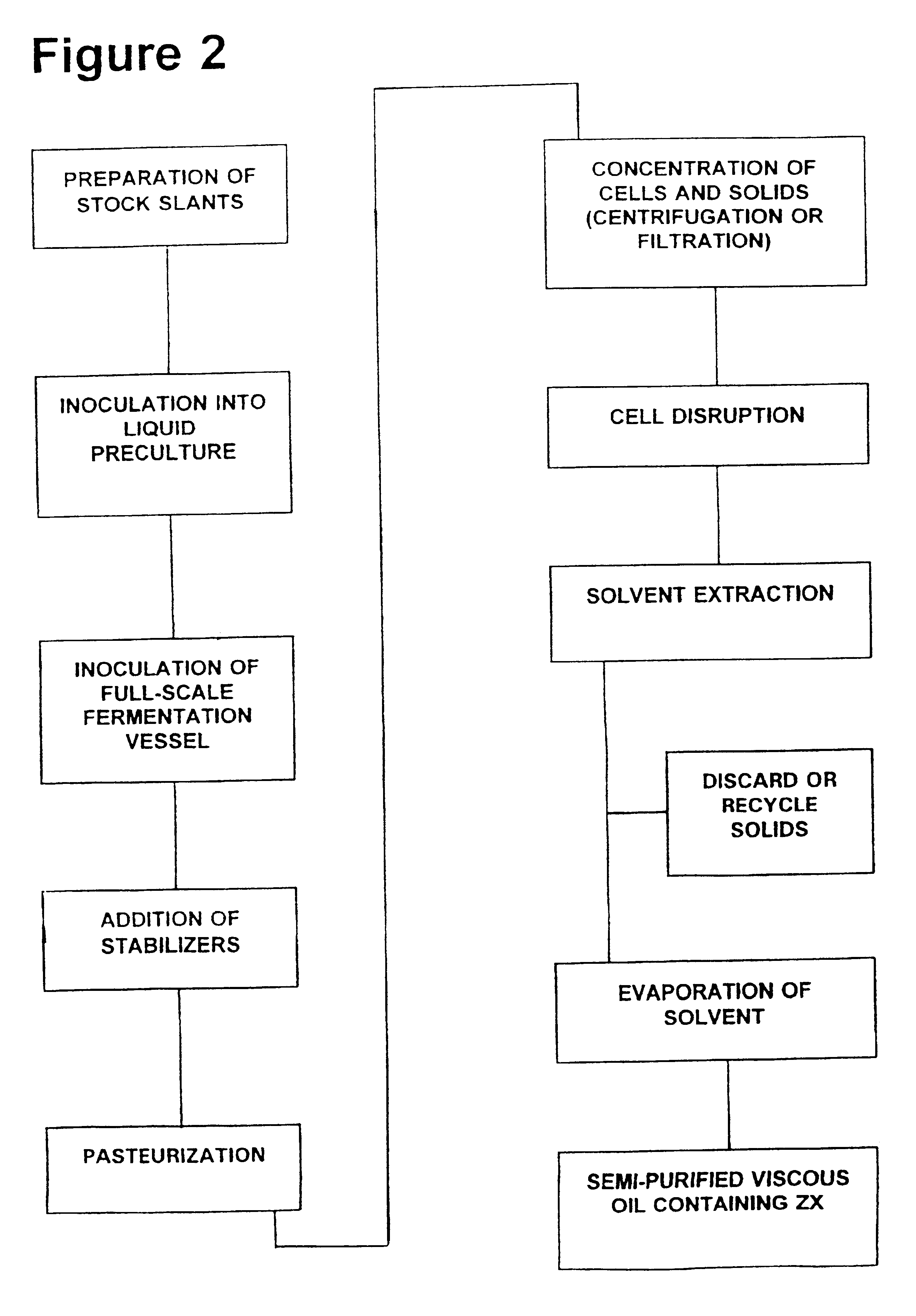
Zeaxanthin formulations for human ingestion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples 6 and 7
describe in vivo retinal tests on Japanese quail, which provide a good animal model for the human macula.
.Iadd.Periodic oral ingestion is the preferred mode of administering zeaxanthin for retinal protection purposes, using ingestion modes such as daily or weekly capsules, or occasional use of zeaxanthin-supplemented food preparations such as margarine. "Periodic" ingestion does not require regular ingestion at fixed intervals (such as daily or weekly pills), but instead refers to occasional, intermittent ingestion which allows a reasonable period of time to elapse between dosages, thereby allowing appropriate and gradual deposition of small quantities of zeaxanthin in the macular tissue. As with any vitamin supplement, a single dosage may be beneficial, but a single dosage will not be as beneficial over a period of years as periodic ingestion of appropriate small dosages. Pharmacodynamic studies on carotenoid uptake in humans and test animals suggest that daily ingestion is prefera...
example 1
Commercial-Scale Fermentation
The nutrient medium that was preferred by the Applicants for lab-scale testing of F. multivorum was identified as nutrient medium E under Example 3 in U.S. Pat. Nos. 5,308,759 (Gierhart 1994) and 5,427,783 (Gierhart 1995). This lab-scale medium contained several ingredients that were expensive and difficult to work with, and substantial work to create a better commercial-scale nutrient medium was carried out after the initial filing date of those applications. The nutrient media that are currently preferred for commercial-scale fermentation have eliminated corn flour and several other ingredients, and contain either high maltose corn syrup of sugar beet molasses at concentrations ranging from 1 to 10% w / v, along with corn steep liquor at 0.5 to 4% w / v; ammonium sulfate heptahydrate at 0.5% w / v; sodium chloride at 0.5% w / v; magnesium sulfate heptahydrate at 0.1% w / v; sodium acetate at 0.1% w / v; ferrous sulfate heptahydrate at 0.001% w / v; yeast extract at ...
example 2
Addition of Stabilizing Agents
Zeaxanthin produced by the fermentation processes of Example 1 needs to be stabilized in order to facilitate subsequent purification and formulation, and to ensure purity. Stabilizing compounds can be added to the F. multivorum cells (or to a cellular extract containing zeaxanthin) at any time during a preparation or purification process; in general, one or more initial stabilizers should be added to the cells while they are still in the fermentation vessel.
Various candidate stabilizers have been tested by the Applicants. The best results obtained to date have used a combination of stabilizing agents, which are mixed together in a small quantity of a suitable solvent (such as about 2 milliliters of ethanol for a 20 liter fermentation vessel) before being added to the cells. The preferred stabilizer mixture contains tertiary butyl hydroquinone (abbreviated as TBHQ; also called 2-(1,1-dimethyl)-1,4-benzenediol) at a quantity which will generate a final co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com