Core-shell oxide material, method for producing the same, and catalyst and method for purification of exhaust gas using the core-shell oxide material
a core-shell oxide and oxide material technology, applied in the direction of metal/metal-oxide/metal-hydroxide catalysts, physical/chemical process catalysts, separation processes, etc., can solve the problems of low oxygen diffusion property, slow oxygen storage/release rate, and deterioration of the core-shell structure, so as to improve the oxygen storage/release capacity, improve the oxygen storage/release rate, and improve the effect of oxygen storage/release ra
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation example 1
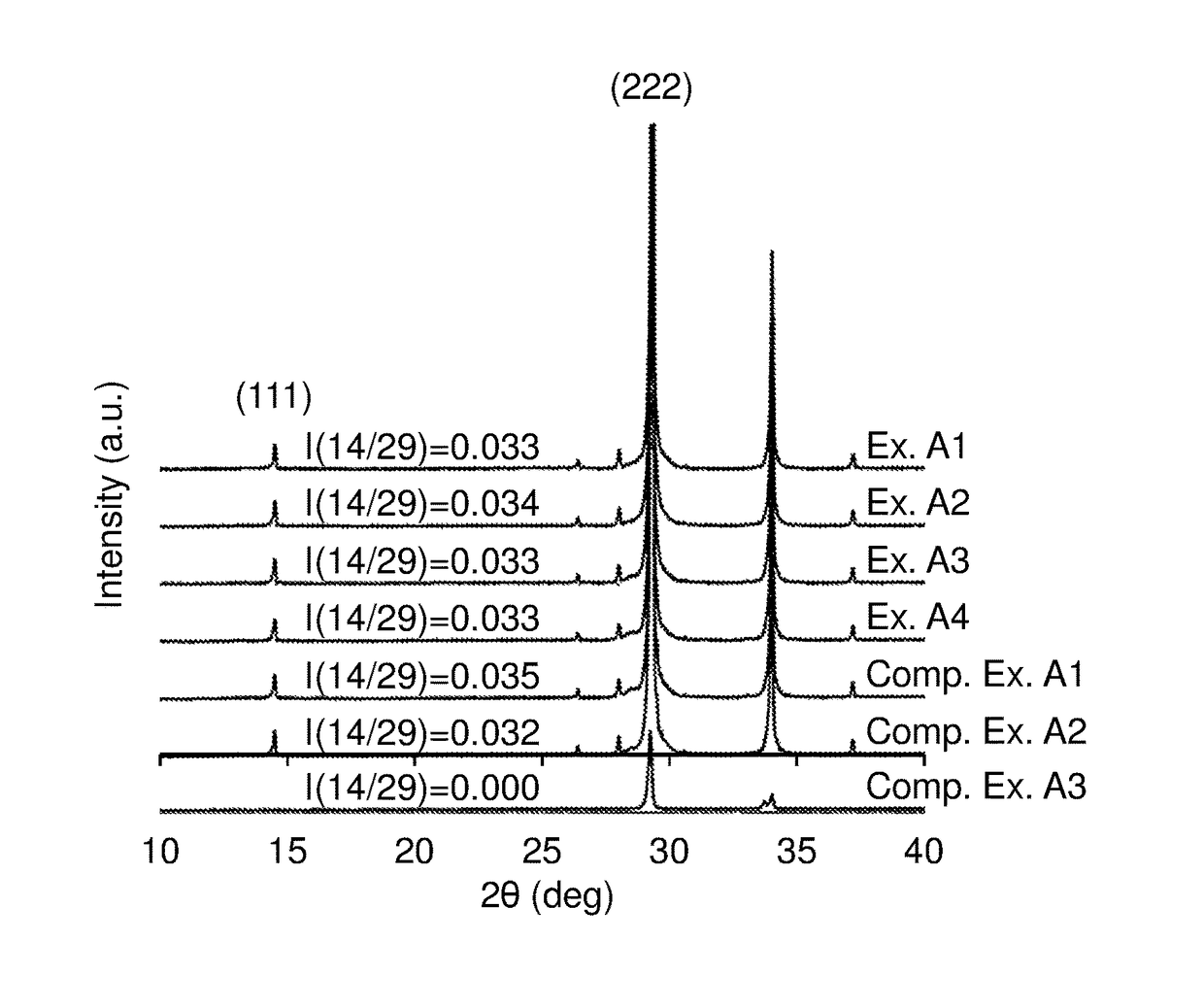
[0071]A ceria-zirconia based solid solution powder in which the content ratio among cerium, zirconium, and praseodymium was 45:54:1 in terms of moles ([cerium]:[zirconium]:[praseodymium]) was prepared as follows. Specifically, first, 442 g of an aqueous solution containing cerium nitrate in an amount of 28% by mass in terms of CeO2, 590 g of an aqueous solution containing zirconium oxynitrate in an amount of 18% by mass in terms of ZrO2, 100 g of an aqueous solution containing praseodymium nitrate in an amount of 1.2 g in terms of Pr6O11, and 197 g of hydrogen peroxide water containing hydrogen peroxide in an amount 1.1 times the molar amount of cerium to be contained were added to 1217 g of an aqueous solution containing ammonia in an amount 1.2 times the neutralization equivalent, so that a coprecipitate was formed. The obtained coprecipitate was centrifuged, and washed with ion-exchange water. Next, the obtained coprecipitate was dried at 110° C. for 10 hours or more, and then ca...
example a1
[0076]An aqueous solution of a La-containing alumina precursor was prepared by dissolving 9.5 mmol of aluminium nitrate and 0.096 mmol of lanthanum nitrate in 200 ml of ion-exchange water. To the aqueous solution of the La-containing alumina precursor, 100 g of the CZP powder obtained in Preparation Example 1 was added and stirred for 15 minutes. The obtained dispersion containing the CZP powder was evaporated to dryness by heating at 200° C. with stirring. The resulting dried product was calcined at 900° C. for 5 hours. The obtained calcination product was ground and sieved such that the particle diameters were 75 μm or less. Thus, a core-shell oxide material powder in which the surface of the CZP powder was coated with an alumina layer containing lanthanum was obtained (the CZP amount: 100 parts by mass, the amount of the alumina coating: 0.5 parts by mass, the amount of the lanthana coating: 0.015 parts by mass).
example a2
[0077]In the same manner as in Example A1, a core-shell oxide material powder in which the surface of the CZP powder was coated with the alumina layer containing lanthanum was prepared (the CZP amount: 100 parts by mass, the amount of the alumina coating: 0.5 parts by mass, the amount of the lanthana coating: 0.015 parts by mass). Next, in the same manner as in Example A1 except that 100 g of this core-shell oxide material powder was used in place of the CZP powder, a core-shell oxide material powder in which the surface of the CZP powder was coated with an alumina layer containing lanthanum was obtained (the CZP amount: 100 parts by mass, the amount of the alumina coating: 1.0 parts by mass, the amount of the lanthana coating: 0.03 parts by mass).
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| 2θ | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com