Crystalline forms of hydroxynorketamine
a technology of hydroxynorketamine and crystalline forms, which is applied in the directions of capsule delivery, organic active ingredients, organic chemistry, etc., can solve the problems of difficult processing into a pharmaceutical formulation, drawbacks of parenteral formulations, and challenges in developmen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
of 2R,6R-hydroxynorketamine hydrochloride
[0198](R)-N-Boc-norketamine (47)
[0199]Boc protection of norketamine was achieved with 95% yield by treating with Boc2O and triethylamine in THF at 70° C. for 16-18 hours.
[0200]1H-NMR (301 MHz, CHLOROFORM-D) δ 7.81 (d, J=6.9 Hz, 1H), 7.26 (d, J=48.2 Hz, 3H), 6.57 (s, 1H), 3.78-3.83 (m, 1H), 2.22-2.41 (m, 2H), 2.02-2.05 (m, 1H), 1.58-1.81 (m, 4H), 1.27 (s, 9H)
[0201]LCMS 20-70% MeCN: 0.1% formic acid / water; short acid methods , C18-CSA, 1.03 min m / z (+ve) 268.1 / 270.1 (loss of boc+H)
[0202](R)-N-Boc-norketamine-6-trimethylsilyl enol ether (48)
[0203]The trimethylsilyl enol ether of Boc protected R-norketamine was achieved with 99% yield by treating with strong base (lithium diisopropylamide (LDA)) in THF at −78° C., taking care to remove trace moisture from the reagents to avoid stalling the reaction.
[0204](2R, 6R)-N-Boc-6-hydroxynorketamine (49)
[0205]Alpha hydroxylation of the trimethyl silyl enol ether of R-norketamine was achieved with 92% yield...
example 2
of Crystalline forms of 2R,6R-hydroxynorketamine Salts
[0214]Methods of Analysis
[0215]X-ray Powder Diffraction (XRPD)—Transmission
[0216]XRPD analysis was carried out on a PANalytical X′pert pro, scanning the samples between 3 and 35° 2θ. The material was gently ground to release any agglomerates and loaded onto a multi-well plate with Kapton or Mylar polymer film to support the sample. The multi-well plate was then placed into the diffractometer and analysed using Cu K radiation (α1 λ=1.54060 Å; α2=1.54443 Å; β=1.39225 Å; α1:α2 ratio=0.5) running in transmission mode (step size 0.0130° 2θ) using 40 kV / 40 mA generator settings.
[0217]X-ray Powder Diffraction (XRPD)—Reflectance
[0218]XRPD analysis was carried out on a Philips X′pert Pro Multipurpose Diffractometer using a spinning stage with autosampler, scanning the samples between 3 and 35° 2θ. The material was loaded onto a circular sample holder and flattened using a glass slide. The sample holder was then loaded into position on the...
example 3
ric Analysis of Crystal Forms of 2R,6R-hydroxynorketamine
[0286]TG / DVA Analysis of 2R,6R-hydroxynorketamine hydrochloride
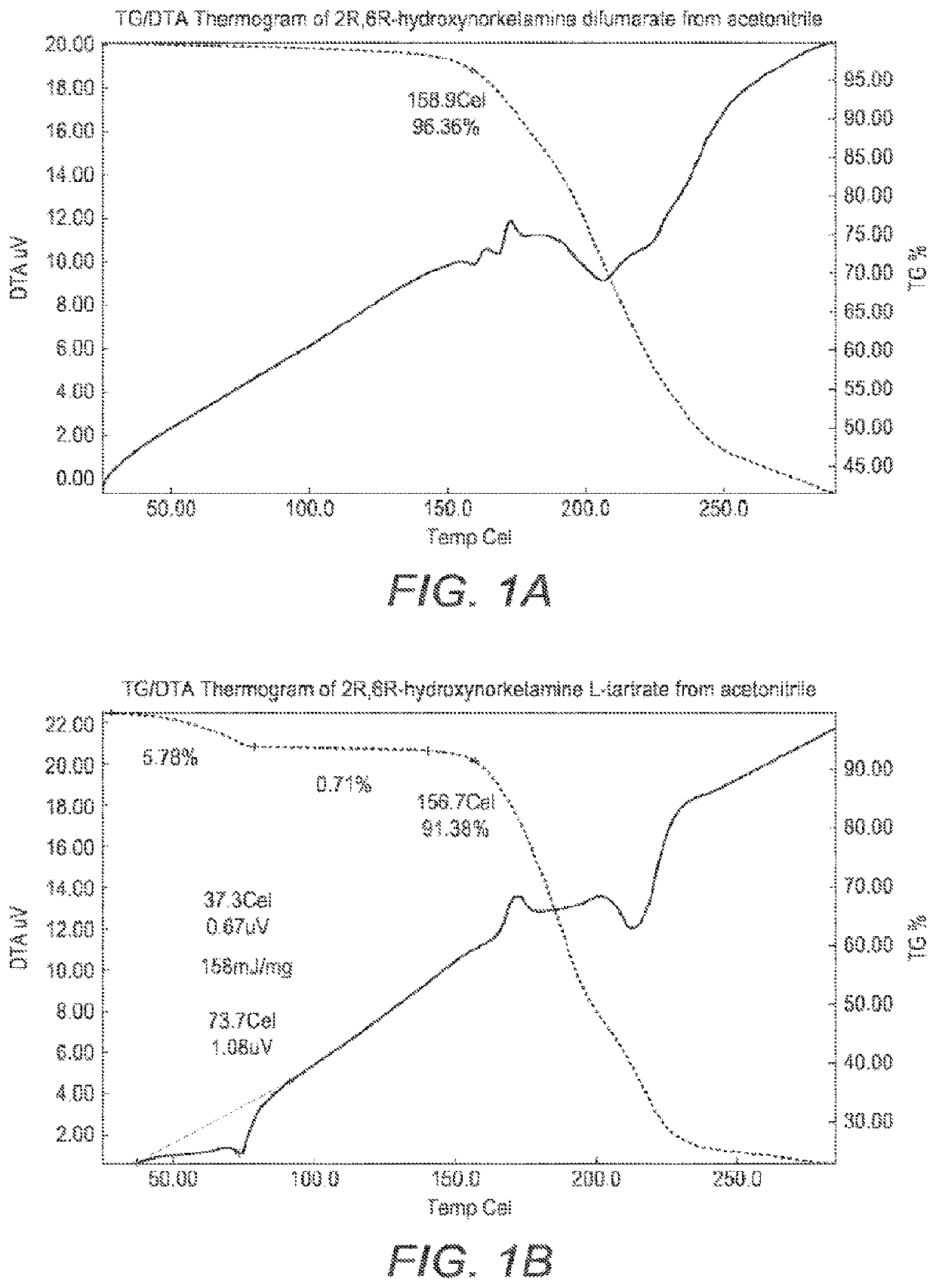
[0287]The TG / DTA Thermogram of the solids recovered from acetone is presented in FIG. 1A. TG / DTA shows that there is a sharp mass loss of 17.3 wt. % with an associated thermal event at 159° C. The sharp mass loss is attributed to loss of bound HCl which would be lost as a gas at that temperature, hence the sharp loss. The 17.3 wt. % loss calculates to 1 equivalent of HCl.
[0288]TG / DVA Analysis of 2R,6R-hydroxynorketamine difumarate
[0289]FIG. 1B presents the TG / DTA thermogram of the solid recovered from acetonitrile. The material degrades above 159° C. There were no thermal events in the DTA.
[0290]The 1H-NMR spectrum of the fumaric acid solid recovered from acetonitrile shows a singlet at 6.6 ppm with an integral of 4.2 protons gives 2 equivalents of fumaric acid per API. The presence of 2 equivalents of fumaric acid suggests the presence of a salt co-crystal.
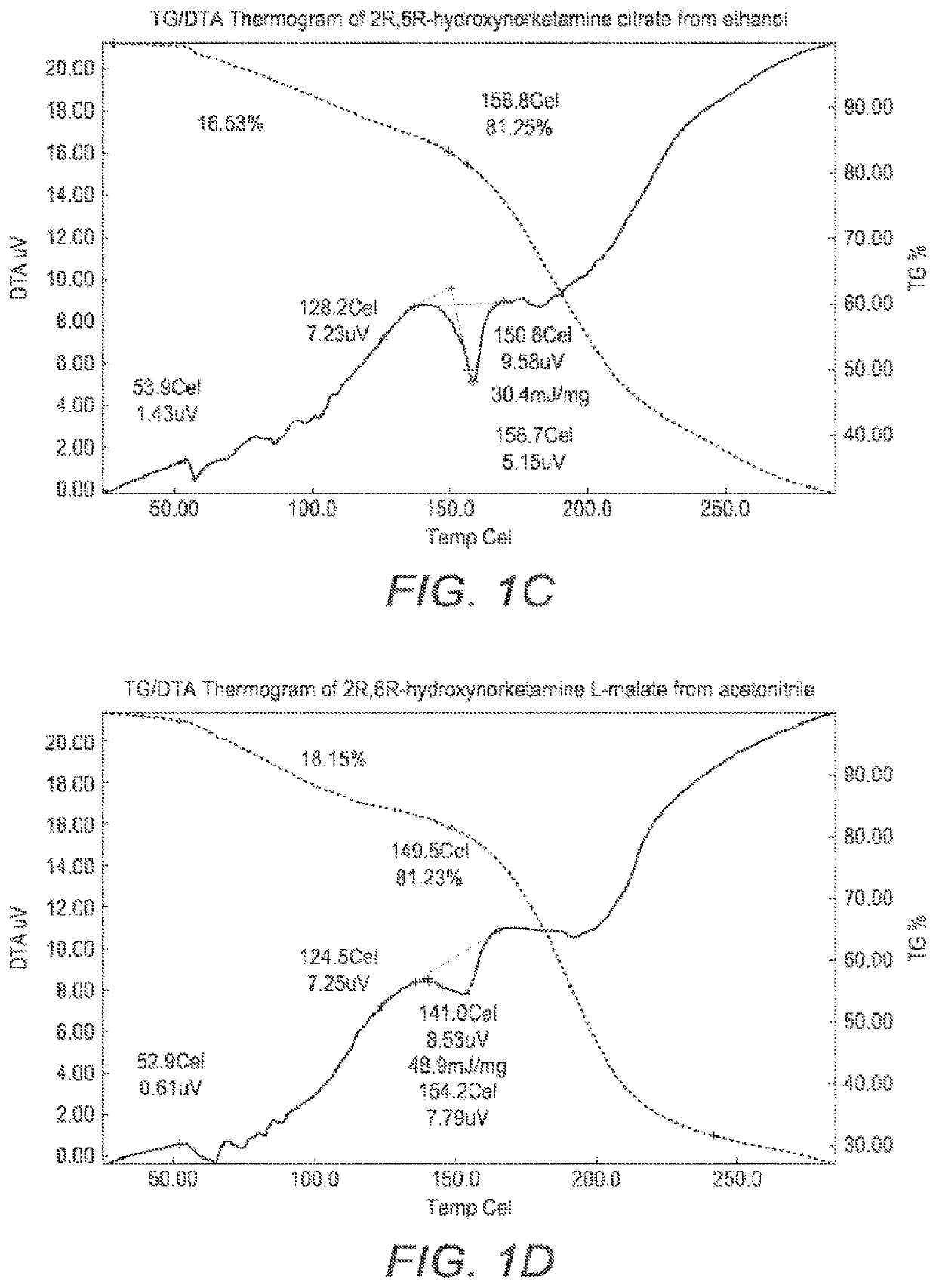
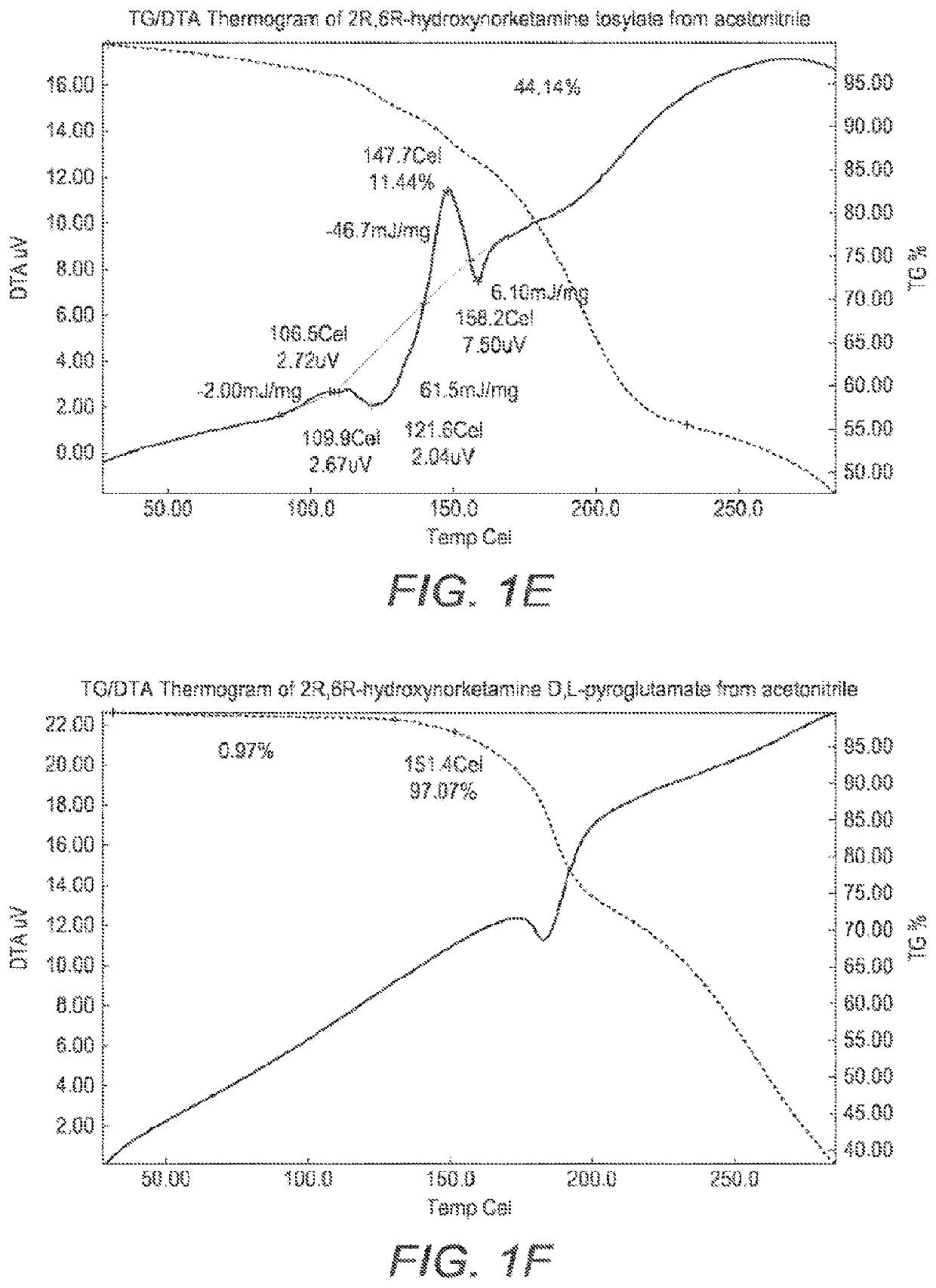
[0291]T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com