Method of producing high bulk density molybdenum oxychloride
a molybdenum oxychloride and high bulk density technology, applied in the direction of chemically reactive gases, coatings, crystal growth processes, etc., can solve the problems of difficult handling, low vapor pressure, and low molybdenum pentachloride (moclsub>5/sub>), and achieve low bulk density, easy handling, and high bulk density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
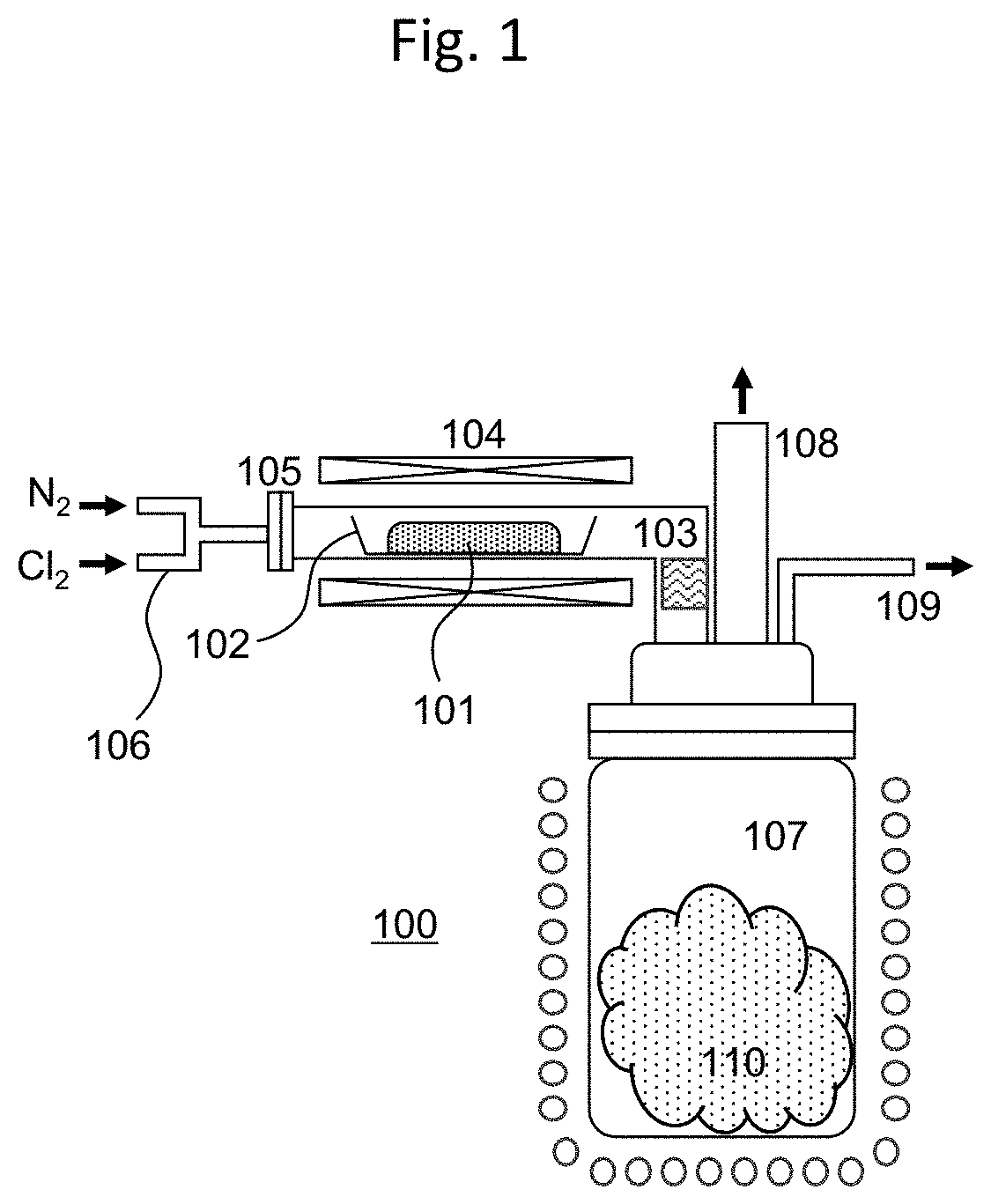
[0064]By using the MoO2Cl2 obtained in Comparative Example 1 as the raw material, the method of the present invention, including sublimation / reaggregation, was applied in a reduced-pressure atmosphere. Here, the device having the configuration of FIG. 2(b) was used to perform the sublimation / reaggregation of the MoO2Cl2. The MoO2Cl2 as the raw material was held in a raw material holding container of a sublimation device, and this was connected to one end of a reaction tube made of glass. The inside of the sublimation device was depressurized by being exhausted from the side while maintaining the other end of the reaction tube in a state where the inside of the tube can be observed from the upper part. In Example 1, the pressure inside the device was set to 1 kPa. In this state, the MoO2Cl2 in the raw material holding container was gradually heated, and held at a heating temperature of 95° C. in which the raw material can be stably sublimated. When this state is maintained, the MoO2C...
example 2
[0065]The pressure inside the device was set to 5 kPa, and the raw material MoO2Cl2 was subject to sublimation / reaggregation in the same manner as Example 1. In Example 2, the temperature that the raw material MoO2Cl2 can be stably sublimated was 117° C., and the heating temperature of the raw material MoO2Cl2 was held at this temperature. Here, the temperature of the reaggregated part was 97° C. In this state, the sublimation / reaggregation of the MoO2Cl2 was performed until the sublimated sediment no longer increased based on visual observation while adjusting the position in the reaction tube where the reaggregated MoO2Cl2 is accumulated. Finally, when the MoO2Cl2 accumulated in the reaction tube was cooled and thereafter collected, it became separated from the inner wall of the reaction tube with relatively weak force, and could be easily recovered. FIG. 5 shows the appearance of the MoO2Cl2 obtained in Example 2. The MoO2Cl2 obtained in Example 2 had a bulk density of 1.1 g / cm3,...
example 3
[0066]The pressure inside the device was set to 20 kPa, and the raw material MoO2Cl2 was subject to sublimation / reaggregation in the same manner as Example 1. In Example 3, the temperature that the raw material MoO2Cl2 can be stably sublimated was 135° C., and the heating temperature of the raw material MoO2Cl2 was held at this temperature. Here, the temperature of the reaggregated part was 115° C. In this state, the sublimation / reaggregation of the MoO2Cl2 was performed until the sublimated sediment no longer increased based on visual observation while adjusting the position in the reaction tube where the reaggregated MoO2Cl2 is accumulated. Finally, when the MoO2Cl2 accumulated in the reaction tube was cooled and thereafter collected, the sediment tended to become hard and affixed to the inner wall of the reaction tube, and much effort was required for collecting the MoO2Cl2 in comparison to Examples 1 and 2. FIG. 6 shows the appearance of the MoO2Cl2 obtained in Example 3. The Mo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pressure | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com