Polymer compositions for 3-d printing and 3-d printers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0101]This example provides a description of examples of polymer compositions of the present disclosure and uses of the polymer compositions.
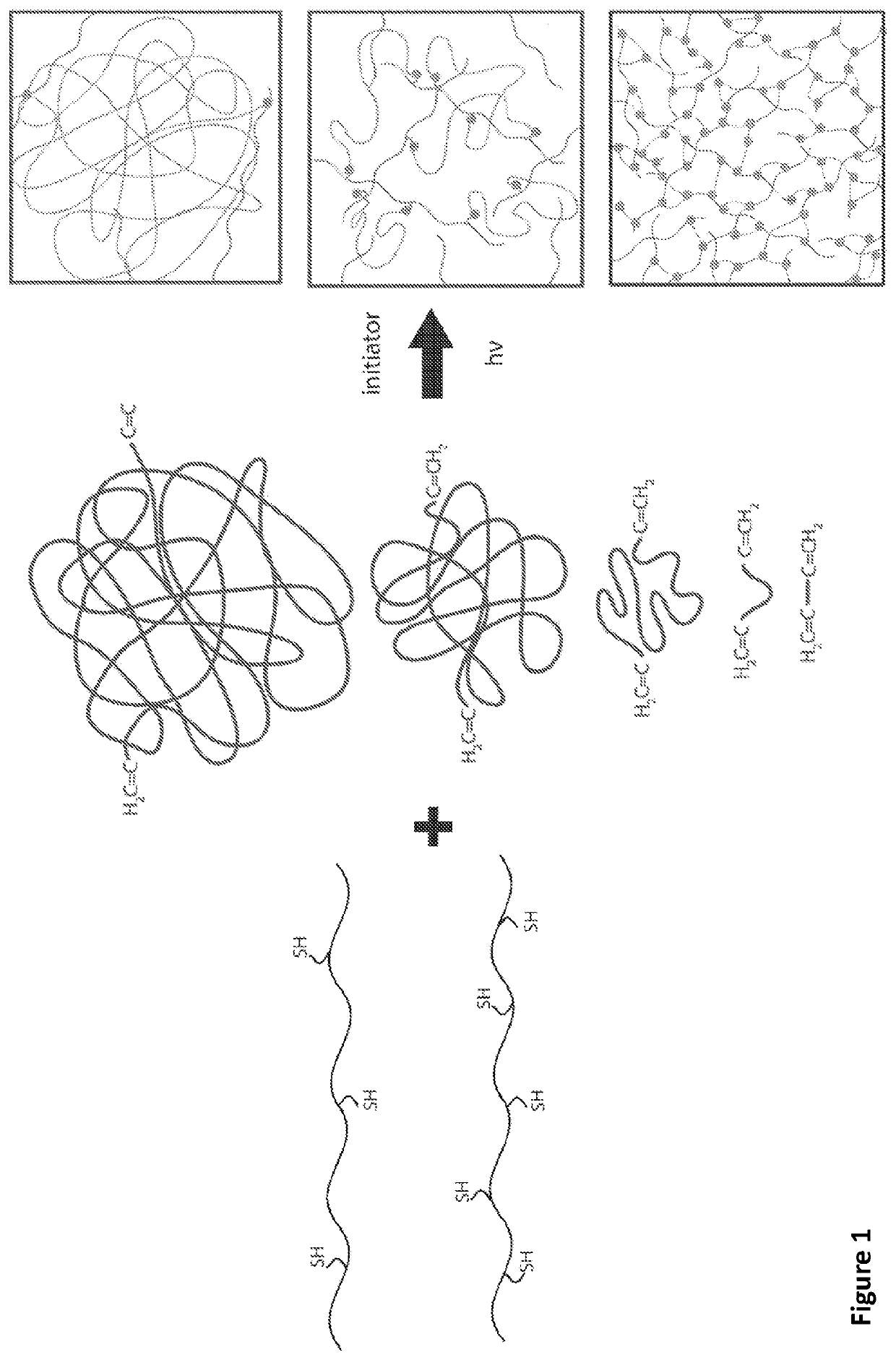
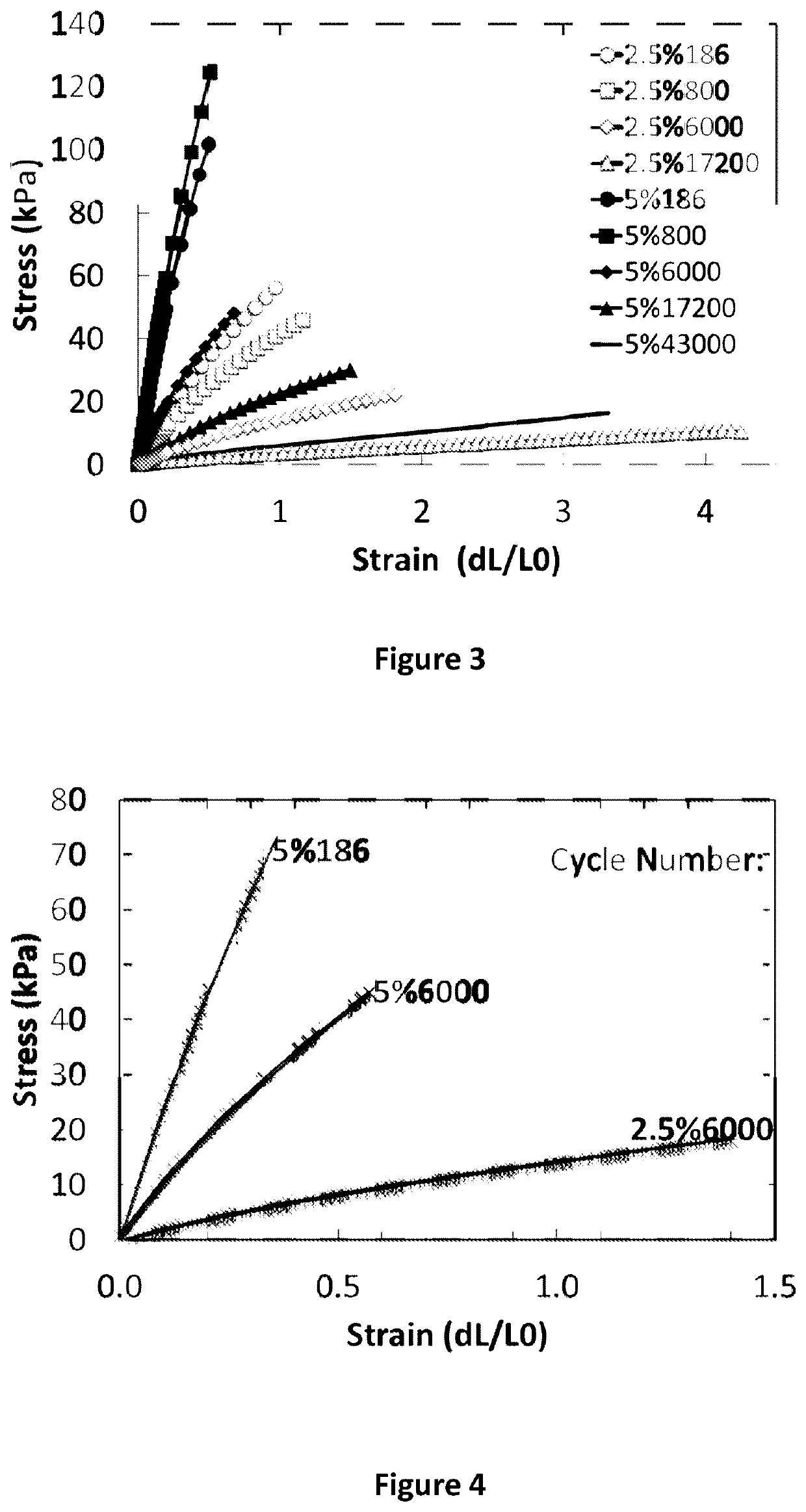
[0102]Described in this example is the rapid fabrication of high-resolution silicone (polydimethylsiloxane) based elastomeric devices via stereolithography. Thiolene click chemistry permits photopolymerization in under 10 seconds and facile tuning of mechanical properties from Young's modulus=6 kPa to 287 kPa, Ultimate elongation=48% to 427%, by controlling the crosslink density and degree of polymerization. From this elastomeric system, we directly fabricate different complaint machines: (i) a living hinge, (ii) a spring and (iii) a pneumatically powered tentacle.
[0103]Thiol-ene chemistry, or alkyl hydrothiolation, is the formation of an alkyl sulfide from a thiol and alkene in the presence of a radical initiator or catalyst. The reaction proceeds rapidly and in such a high yield as to be widely regarded as a form of “click-chemistry.” An idea...
example 2
[0112]This example provides a description of examples of polymer compositions of the present disclosure and uses of the polymer compositions.
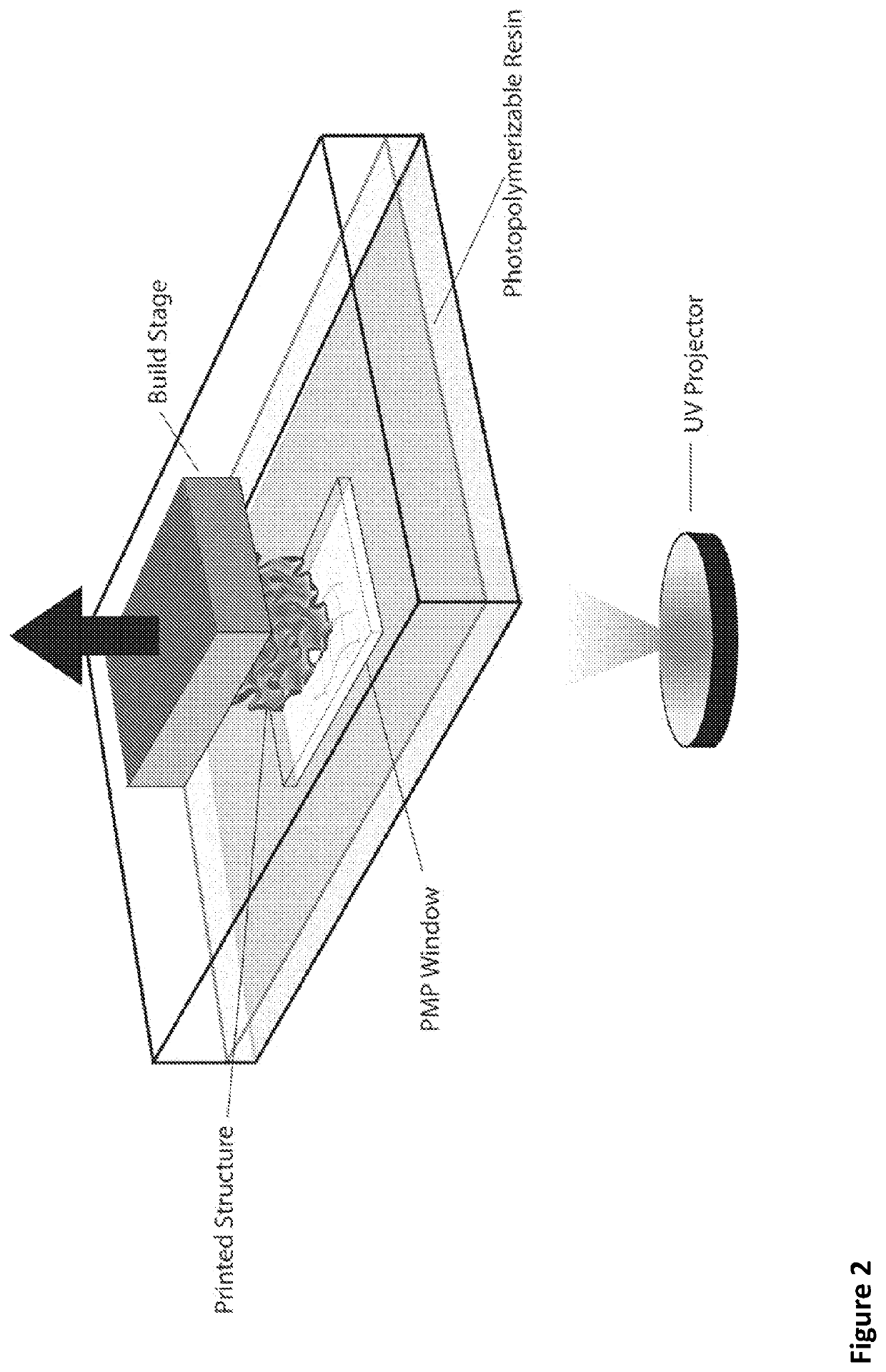
[0113]Described is a low-cost build window substrate that enables the rapid fabrication of high resolution (˜50 μm) silicone (polydimethylsiloxane) based elastomeric devices using an open source SLA printer. Our thiol-ene click chemistry permits photopolymerization using low energy (He−2) optical wavelengths (405 nmult<4 is achievable through appropriate selection of the two primary chemical constituents (mercaptosiloxane, M.S., and vinylsiloxane, V.S.). Using this chemo-mechanical system, we directly fabricated compliant machines, including an antagonistic pair of fluidic elastomer actuators (a primary component in most soft robots). During printing, we retained unreacted pockets of M.S. and V.S. that permit autonomic self-healing, via sunlight, upon puncture of the elastomeric membranes of the soft actuators.
[0114]Ember™ by Autodesk, a commer...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com