Chiral binaphthopyran photochromic compound as well as preparation method and application thereof
A photochromic and compound technology, applied in chemical instruments and methods, color-changing fluorescent materials, organic chemistry, etc., can solve the problems of slow fading rate, lack of optical activity, slow fading, etc., and achieve rapid discoloration and fading speed, Wide application prospects and excellent fatigue resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
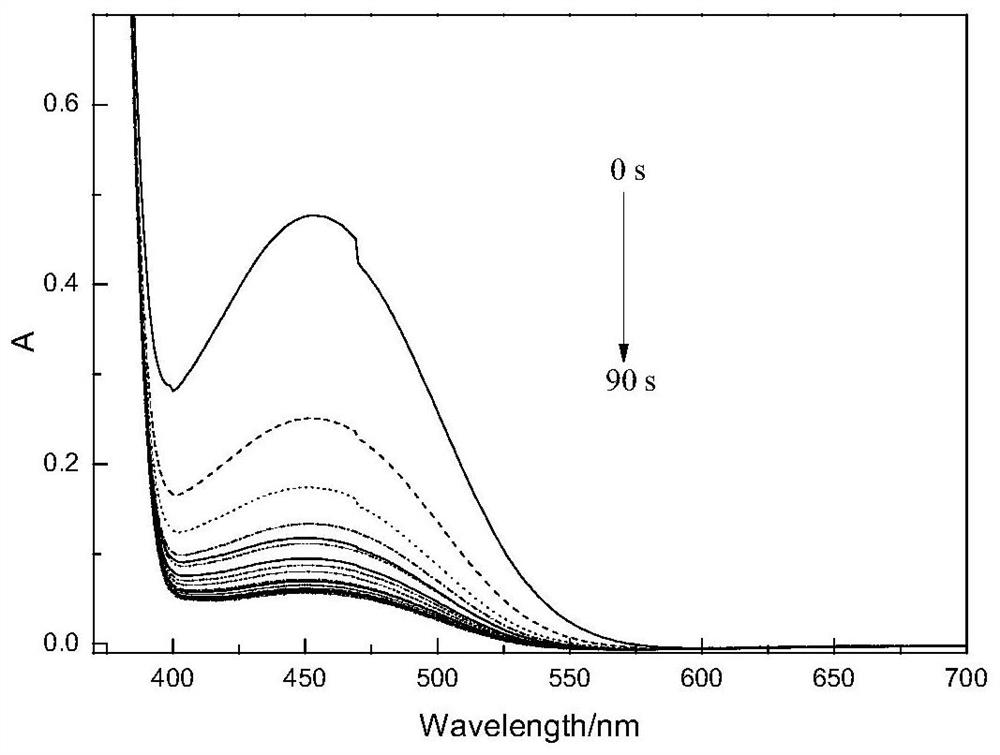
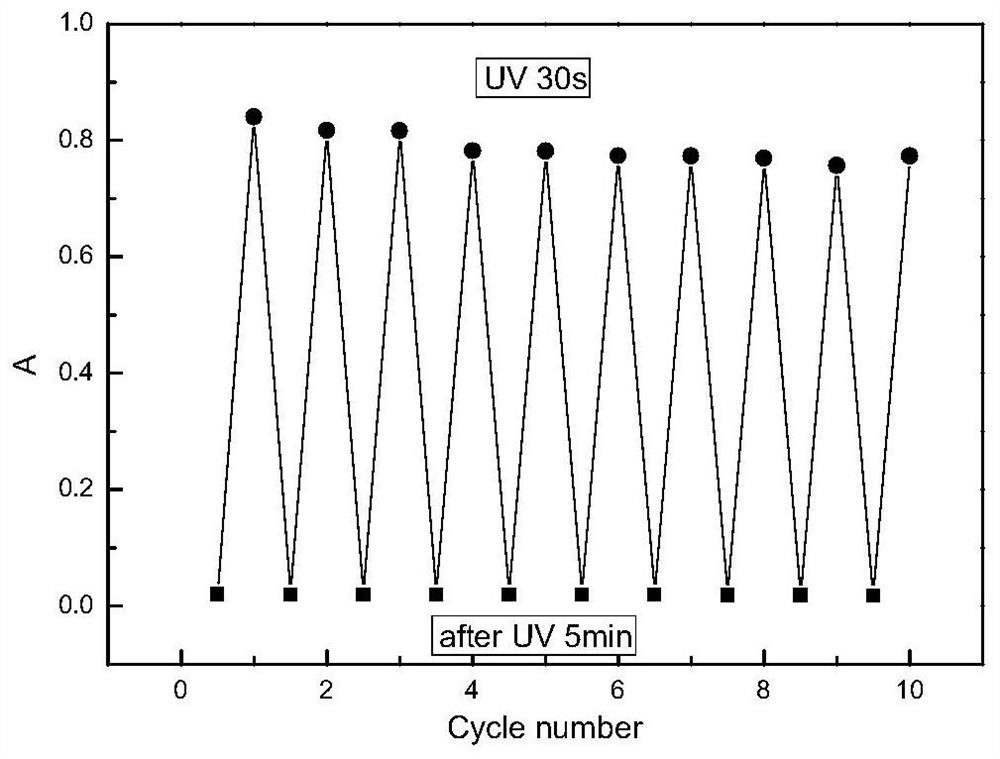
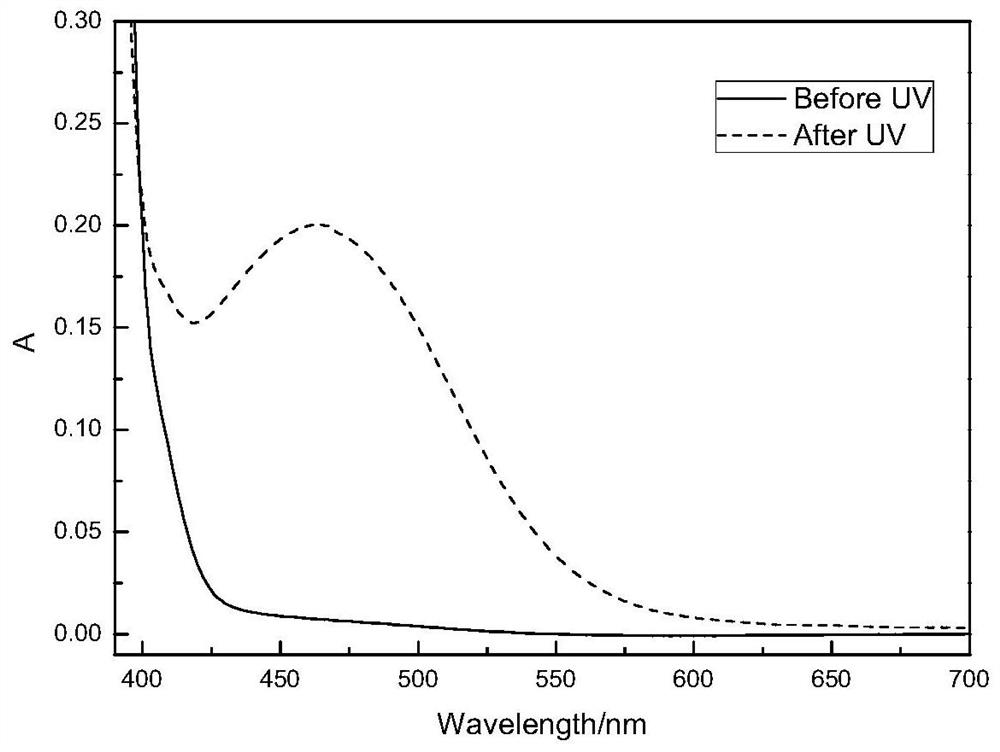
Image
Examples
Embodiment 1
[0031] Embodiment 1: the preparation of photochromic compound (R)-Ia
[0032] Step 1: the preparation of compound (R)-2, the reaction formula is as follows:
[0033]
[0034] N 2 Under protection, chiral 9-bromo-dinaphtho[2,1-d:1',2'-f][1,3]dioxepin(R)-1 (1.90g , 5.09mmol) and tetrahydrofuran (15mL). The reaction system was cooled to -78°C and n-butyllithium (1.6M, 4.85ml, 7.76mmol) was added slowly. Slowly warm up to 0°C and stir for 1.5h. Cool to -78°C and add trimethyl borate (1.20 mL, 10.8 mmol). Stir at room temperature for 18h, add hydrogen peroxide (30%, 1.6ml), heat to 50°C and stir for 6h. Cool to room temperature, add saturated Na to the reaction solution 2 SO 3 solution (20 mL), and extracted with ethyl acetate (50 mL×2). Combine the organic phases and wash with anhydrous Na 2 SO 4 dry. The solvent was removed by concentration, and the residue was purified by silica gel column chromatography (petroleum ether / ethyl acetate=50:1) to obtain a reddish-brow...
Embodiment 2
[0042] The preparation of embodiment 2 photochromic compound (R)-Ib, reaction formula is as follows:
[0043]
[0044] Into a 100 mL round bottom flask was added (R)-2 (0.24 g, 0.77 mmol), toluene (20 mL), 1-(4-biphenyl)-1-phenylprop-2-yn-1-ol ( 0.28g, 1.00mmol) and 2 drops of dodecylbenzenesulfonic acid. Heated to 40°C for 3h. After the reaction was completed, the reaction solution was concentrated under reduced pressure to remove the solvent. The residue was separated by silica gel column chromatography (petroleum ether / ethyl acetate=80:1) to obtain a yellow solid product (R)-Ib with a yield of 86%.
[0045] The proton nuclear magnetic resonance spectrum characterization data of (R)-Ib is: 1 H NMR (400MHz, CDCl 3 )δ(ppm)8.02(d,J=8.0Hz,1H),7.90–7.77(m,2H),7.57–7.37(m,10H),7.37–7.26(m,7H),7.25–7.00(m, 4H), 6.95(d, J=8.0Hz, 1H), 6.25(d, J=10Hz, 1H), 5.54(s, 2H).
[0046] The carbon nuclear magnetic resonance spectrum characterization data of (R)-Ib is: 13 C NMR (100MH...
Embodiment 3
[0047] The preparation of embodiment 3 photochromic compound (R)-Ic, reaction formula is as follows:
[0048]
[0049] Into a 100 mL round bottom flask was added (R)-2 (0.24 g, 0.77 mmol), toluene (20 mL), 1,1-bis(4-methoxyphenyl)prop-2-yn-1-ol ( 0.27g, 1.00mmol) and 2 drops of dodecylbenzenesulfonic acid. Heated to 40°C for 3h. After the reaction was completed, the reaction solution was concentrated under reduced pressure to remove the solvent. The residue was separated by silica gel column chromatography (petroleum ether / ethyl acetate=50:1) to obtain the pink product (R)-Ic with a yield of 79%.
[0050] The characterization data of (R)-Ic proton nuclear magnetic resonance spectrum is: 1 H NMR (400MHz, CDCl 3 )δ(ppm)8.10(d,J=8.0Hz,1H),7.95–7.89(m,2H),7.52–7.39(m,6H),7.38–7.28(m,5H),7.01–6.95(m, 1H),6.92–6.87(m,2H),6.82–6.77(m,2H),6.25(d,J=10.0Hz,1H),5.64(s,2H),3.81(s,3H),3.75(s ,3H).
[0051] The carbon NMR spectrum characterization data of (R)-Ic is: 13 C NMR (100...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com