Compositions for treating wounds
a technology for wounds and compositions, applied in drug compositions, peptide/protein ingredients, bandages, etc., can solve the problems of ulceration, poor wound healing, and loss of sensation, and achieve the effects of reducing the number of ulcers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
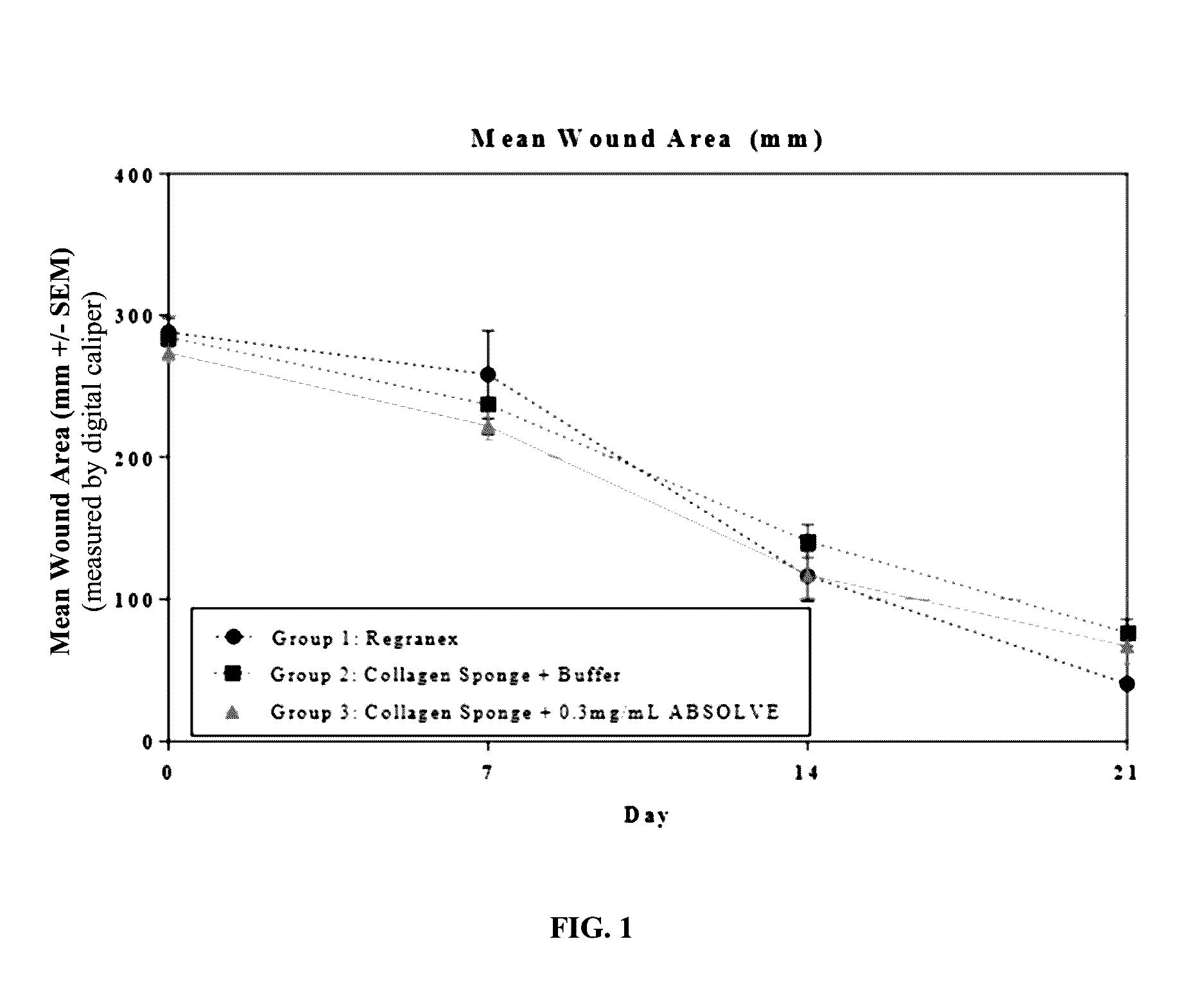
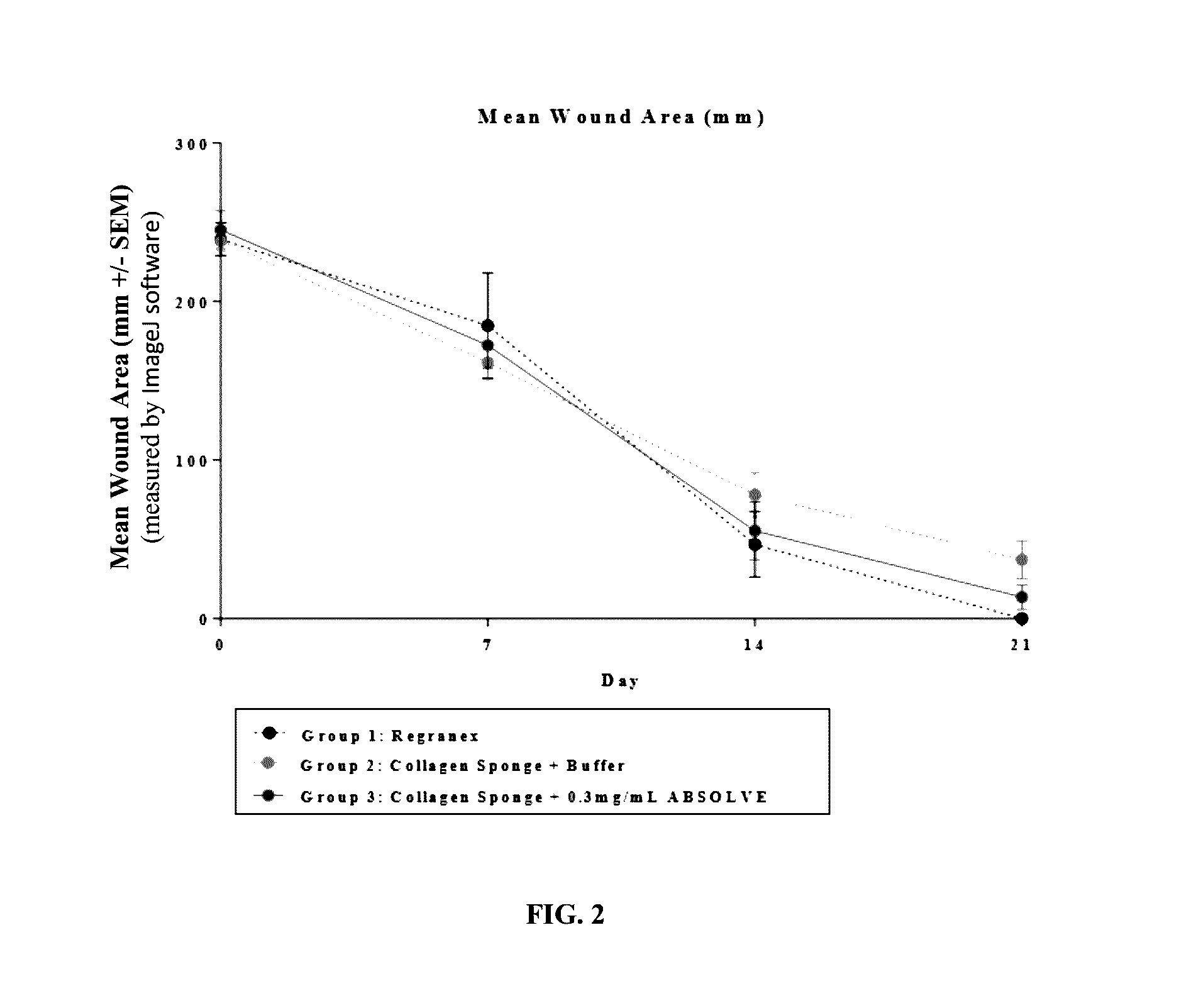
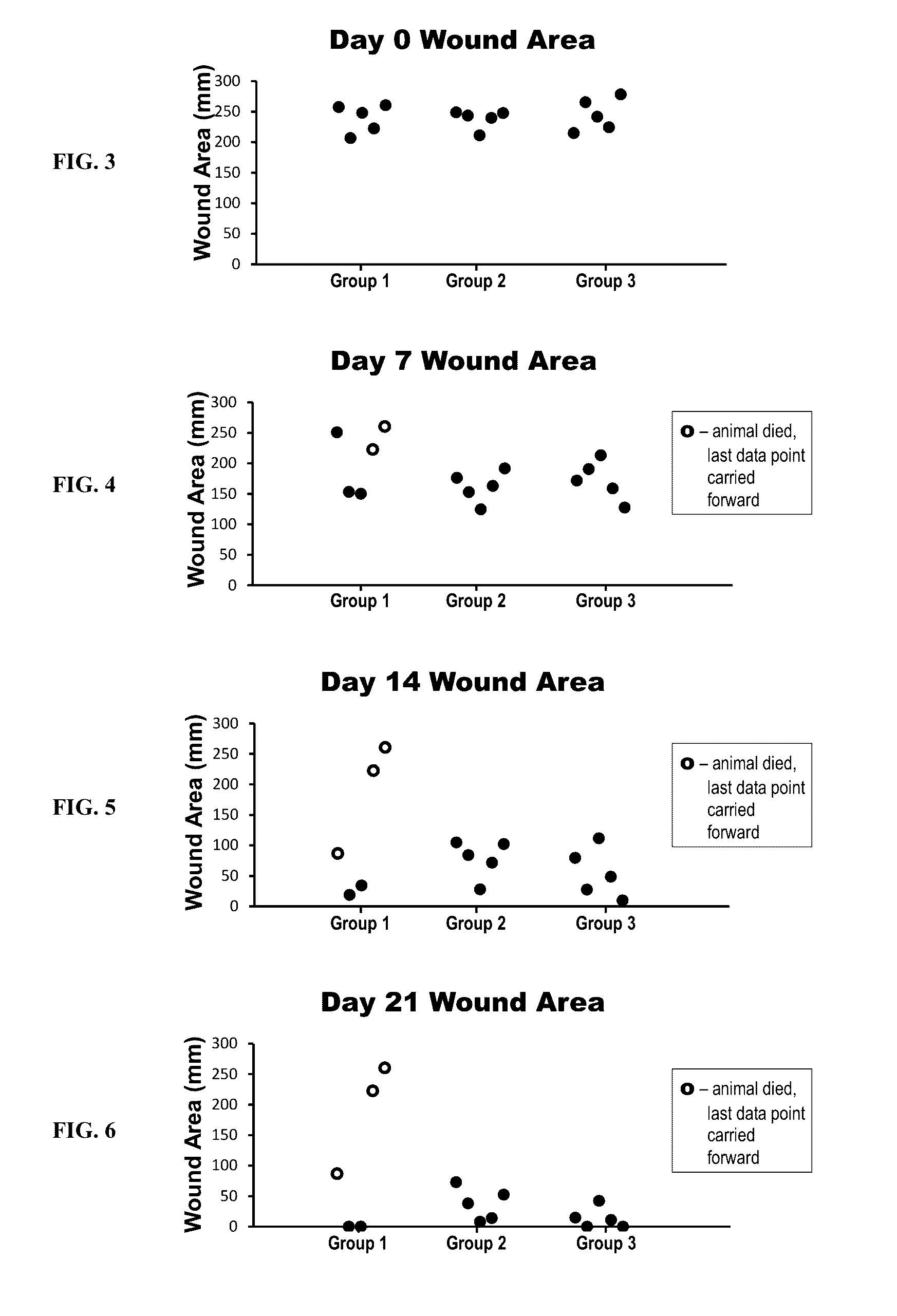
[0133]The efficacy of a collagen wound dressing containing 0.3 mg / ml recombinant human platelet derived growth factor-BB (rhPDGF-BB) was evaluated in the treatment of surgically induced full thickness wounds in mice rendered diabetic by a mutation in the leptin receptor (db / db).
A. Study Design
[0134]Fifteen (15) male C57 / B6 (Leprdb) db / db mice with an average starting body weight of 41.46 g were obtained from Jackson Laboratory (Bar Harbor, Me.) strain code 000642. Animals were acclimatized prior to study commencement. During this period of 3 days, the animals were observed daily in order to reject animals that presented in poor condition.
[0135]During the study all animals were single housed under identical conditions in disposable cages. The study was performed in animal rooms provided with HEPA-filtered air at a temperature of 70° F.+ / −5° F. and relative humidity of 50%+ / −20%. Animal rooms were set to maintain a minimum of 12 to 15 air changes per hour. The room was on an automatic...
example 2
Prophetic
[0177]A study is conducted to demonstrate the efficacy of the novel therapeutic compositions and wound treatment methods described herein. The same study design outlined for Example 1 is also used for this study including the db / db mouse model with five test groups a standard of care group (saline moistened gauze), a Regranex group, a collagen sponge group, and two groups utilizing treatment compositions in accordance with the present invention comprising PDGF-BB and a collagen sponge. As detailed below, however, the frequency of the dosing is changed in this study. The study is also designed so that the total dose of PDGF delivered over the course of the study in both the Regranex group and the collagen sponge / PDGF-BB groups is the same.
A. Experimental Design
[0178]The experimental design is refined by the results of the study described in Example 1, however it is anticipated that the number of animals per group are greater (i.e. eight) and the study duration is longer, i.e...
example 3
Prophetic
[0183]A randomized clinical trial is conducted to assess the effectiveness of various compositions of rhPDGF-BB and collagen as compared to standard of care (consisting of moist wound healing with removal of excess wound exudate, debriding necrotic tissue, off-loading of pressure, saline moistened gauze, antibiotics if needed and wound dressing) and Regranex in the treatment of chronic diabetic foot ulcers. Table 4 below summarizes the study design. For each arm of the study (1-37) the product is applied at the dosage and frequency indicated in Table 4 for up to 20 weeks or until complete wound closure. Regranex is applied in accordance with its approve US labeling. rhPDGF-BB / collagen compositions are applied in accordance with the procedures (steps 1-5) described above in paragraph 50.
[0184]The outcome measures for the study are:[0185]Incidence of complete wound closure.[0186]Time to achieve complete wound closure.[0187]Percentage reduction in total ulcer surface area at e...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore sizes | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| pore size distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com