Compounds with super-aspirin effects
a technology of compound and aspirin, which is applied in the field of compound with super-aspirin effects, can solve the problems of reducing the dose, poor adhesion, and inability to solve the aspirin resistance problem of enteric coated aspirin, and achieves the effects of reducing low density lipoprotein cholesterol and triglycerides, reducing hdl, and inhibiting tcipa
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0092]The present inventors have demonstrated that the compounds used within the context of the present address aspirin resistance problems in 3 major ways:
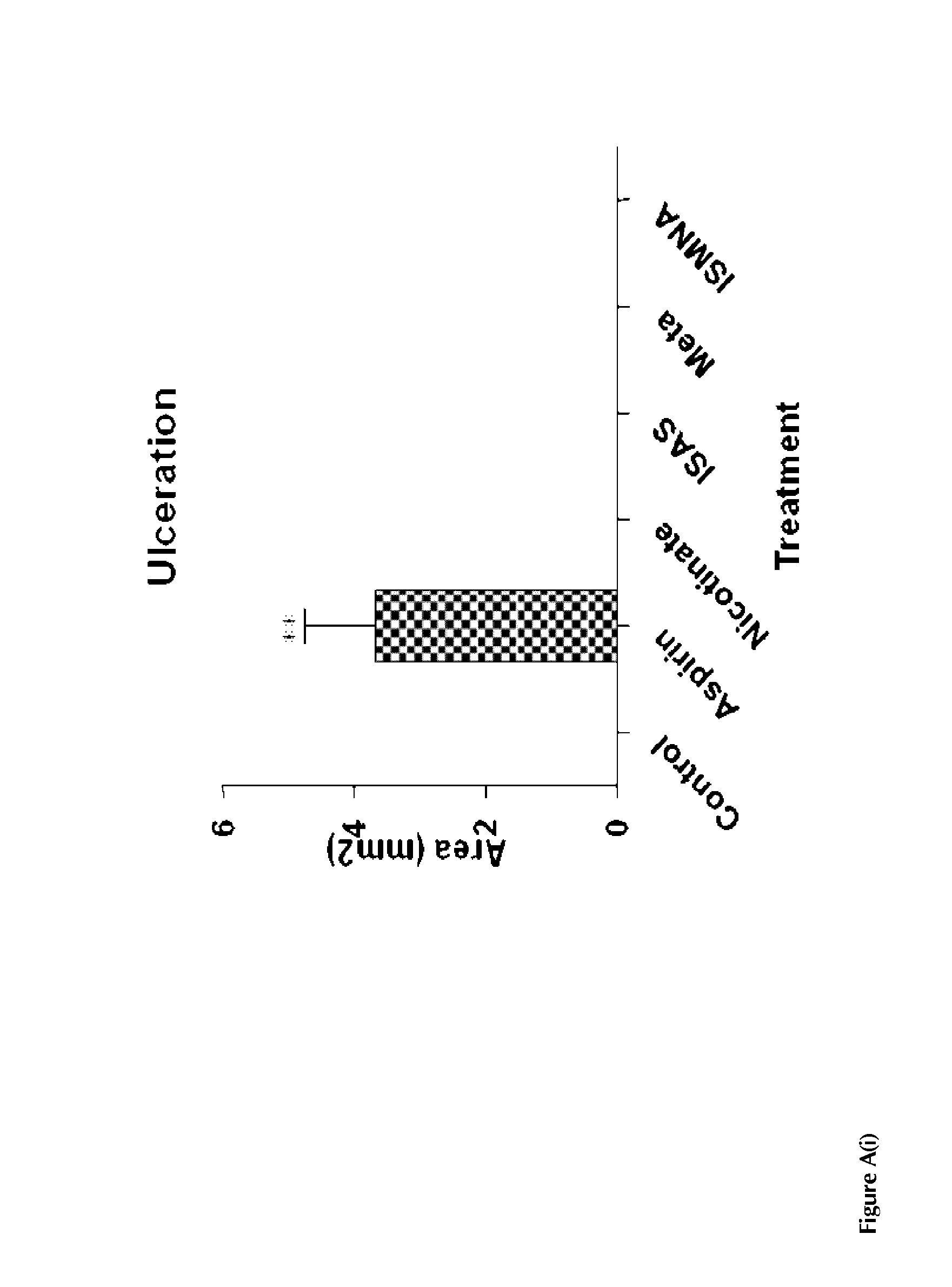
(i) the prodrug compounds used herein demonstrates significantly better GI tolerability when compared to aspirin;
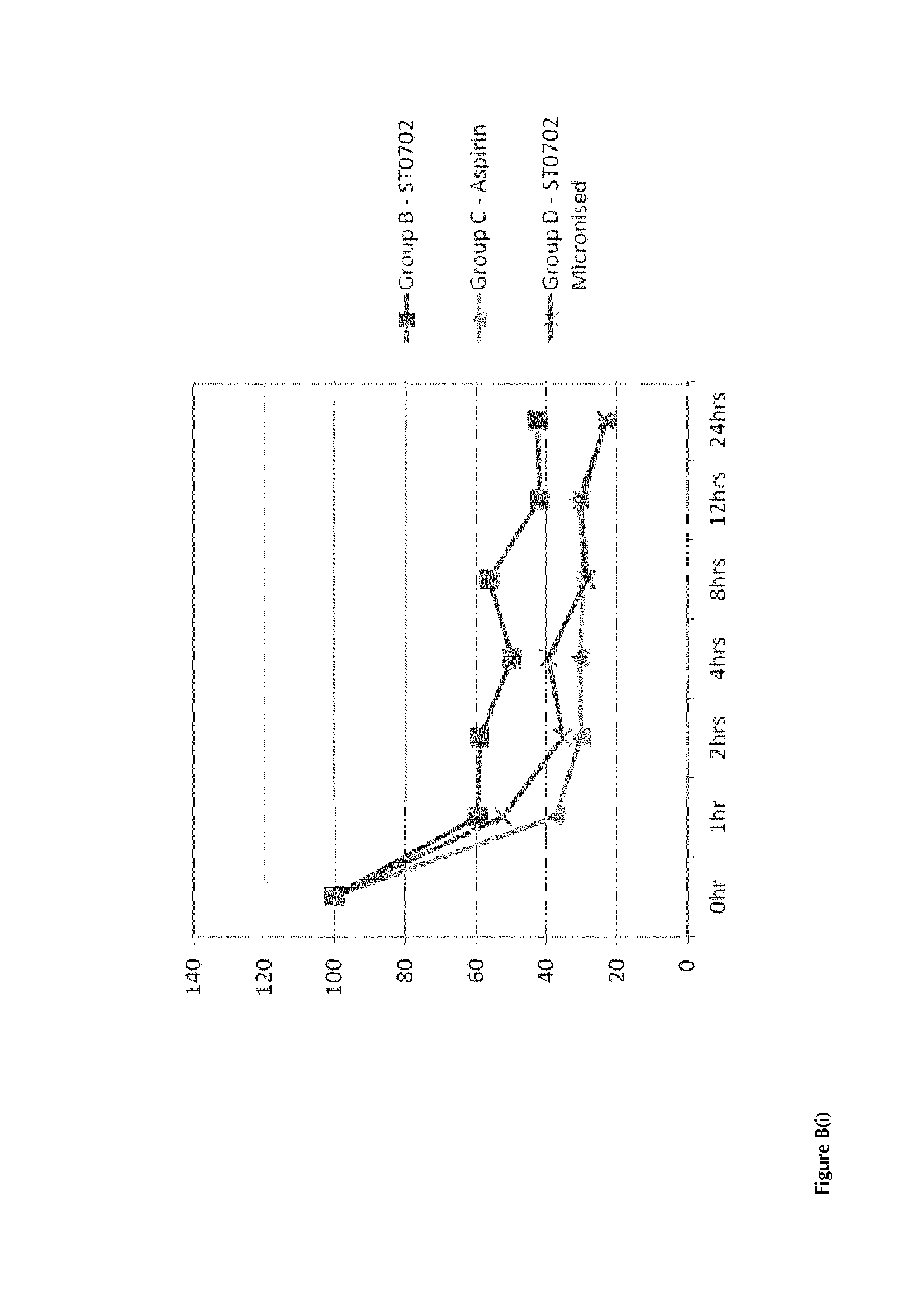
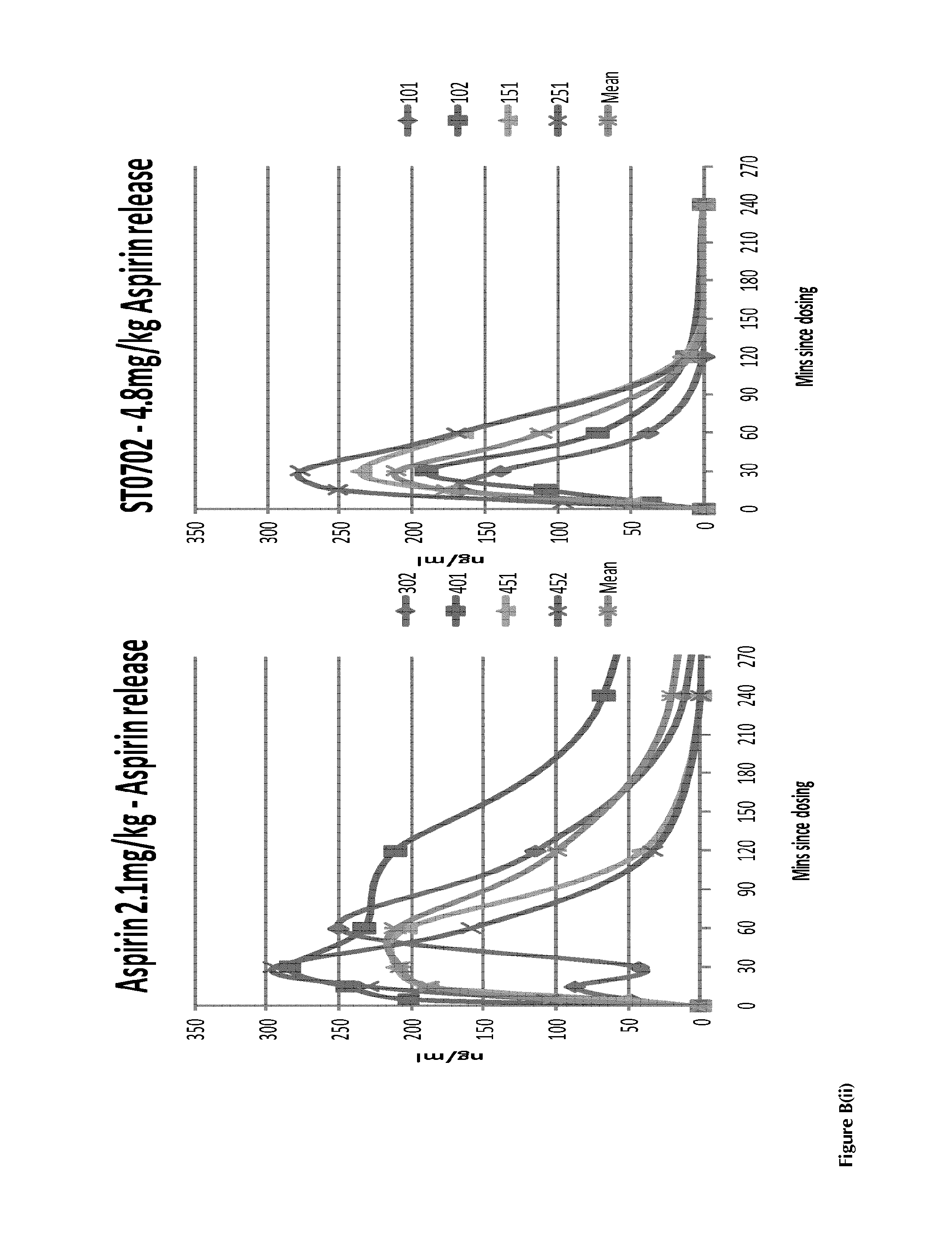
(ii) in the case of ST0702 (ISANA or the “Nicotinate”), data obtained in a Non-Human Primate study indicate that there are surprisingly good effects of the drug in-vivo; and
(iii) in the case of the prodrugs used within the context of the present invention, and exemplified by the data on the nicotinate compound ST0702, there are advantageous and desirable non-aspirin antiplatelet effects produced by a metabolite of the prodrug compounds.
[0093]It has been found that because platelets are activated by multiple pathways, these non-aspirin antiplatelet effects supplement the aspirin effects to reduce platelet activation, particularly in disease states with high levels of inflammation or in patients with genetic polymorphism...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration response curves | aaaaa | aaaaa |
| Weight | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com