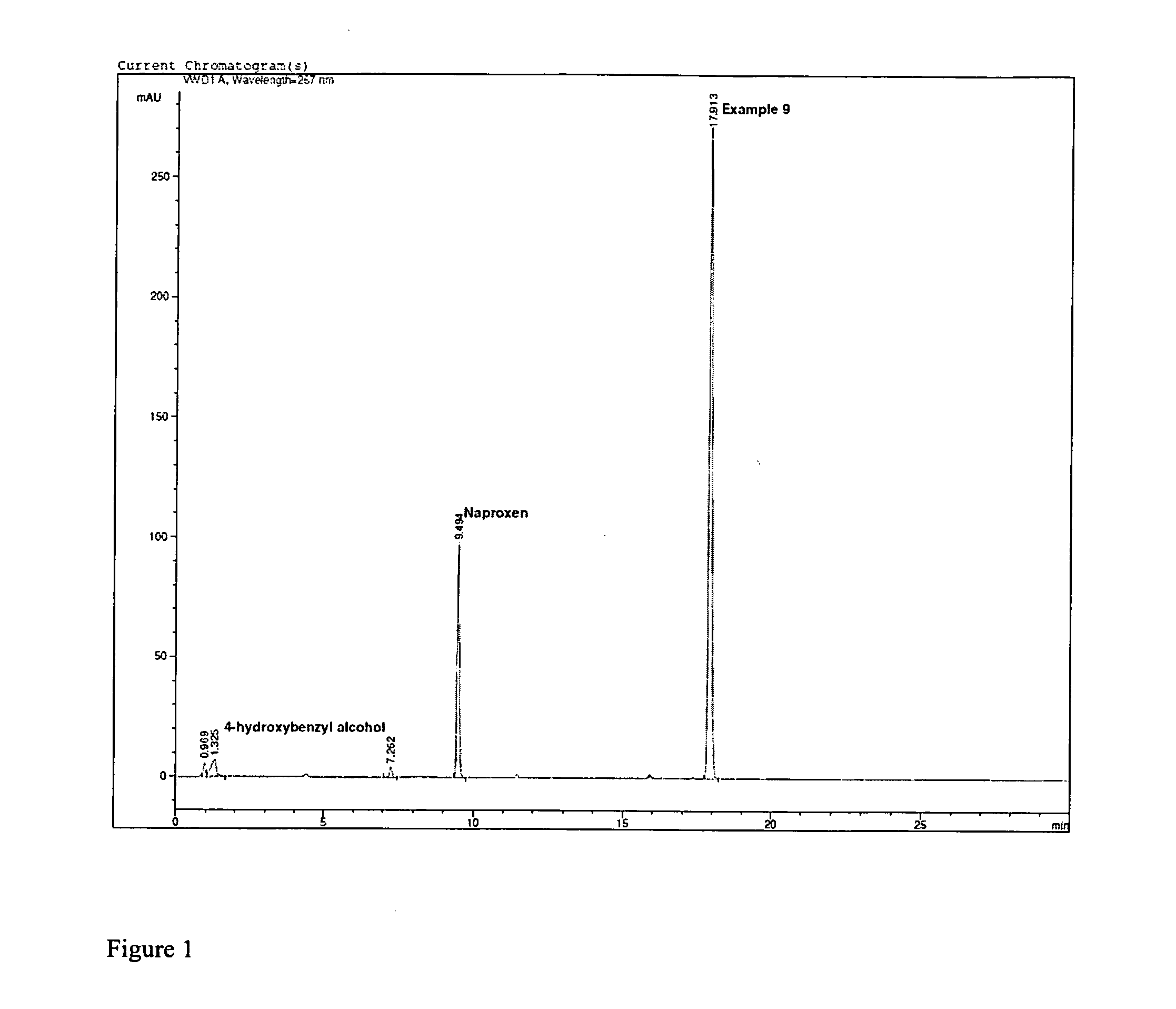
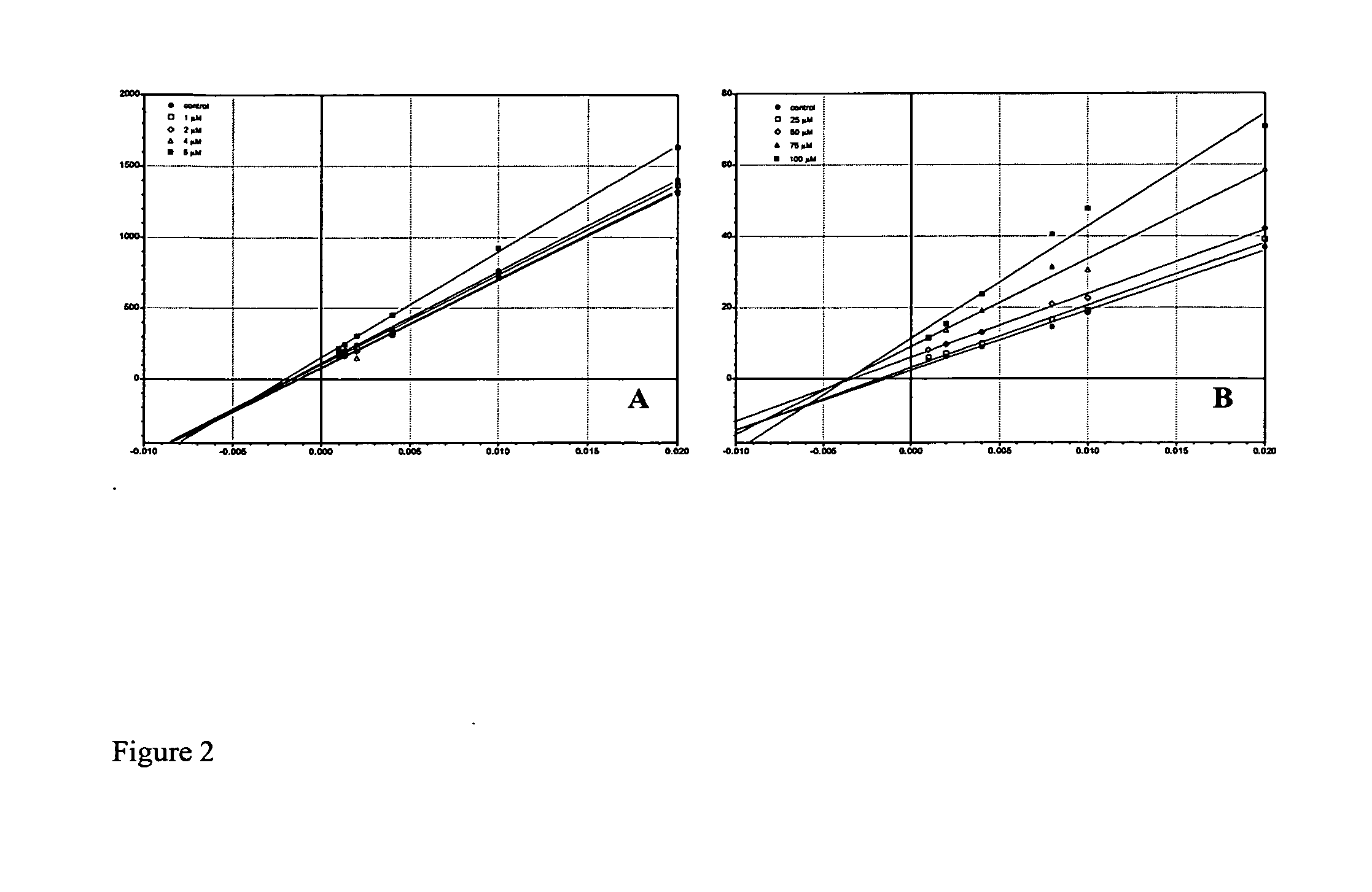
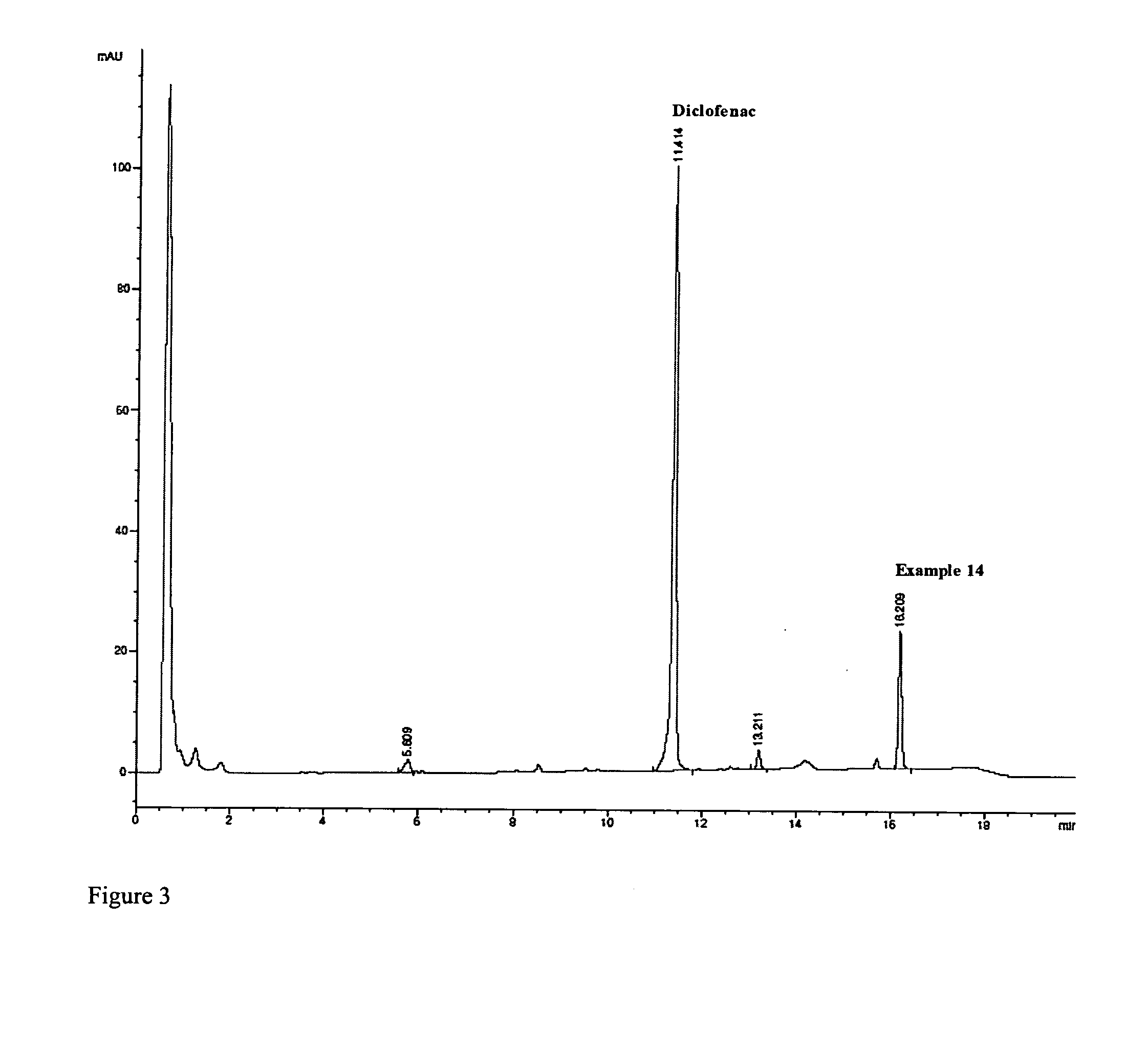
Unique Dual-Action Therapeutics
a dual-action, therapeutic technology, applied in the direction of biocide, application, drug composition, etc., can solve the problems of inability to independently liberate two moieties in vivo, adverse gastrointestinal effects of indomethacin and other nsaids either in prophylaxis or in therapy risks, allergic reactions, and sometimes severe ulcerations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Methods
[0034]All reactants and solvents were of the highest purity commercial grade and were employed without further purification. The suppliers of uncommon reactants are indicated for those reactions and assays reported herein which employ a specialized reagent. All reactions were performed in oven-dried apparatus. 1H NMR and 13C NMR, spectra were recorded on a 500 MHz (Bruker) multinuclear spectrometer and chemical shifts are reported as ppm. All thin layer chromatography (TLC) was performed on Analtech silica gel plates (250 microns). Elemental analyses were performed at Quantitative Technologies (QTI), Inc. The 2-(2-methoxynaphthalene-6-yl)propanoic acid (naproxen) used in this work was the (S)-enantiomer. All other reagents were used as racemates.
Examples by METHOD A
[0035]By METHOD A, an NSAID converted to its acid chloride is coupled to 4-hydroxybenzaldehyde, the aldehyde reduced to a benzyl alcohol, and that alcohol condensed with an appropriate chloroformate (...
example 2
Preparation of 2-(4-Isobutylphenyl)propanoyl Chloride [ibuprofen acid chloride]
[0037]
[0038]A silicone oil bath was heated to 130° C. A reaction set-up was prepared consisting of a 200 mL round bottom flask with condenser and rubber septum-capped joints. After mixing the reactants as described below, the flask and contents were placed in the oil bath. Thionyl chloride (29.7 g, 0.25 mol) was added via glass syringe to 2-(4-isobutylphenyl)propionic acid (10.3 g, 0.05 mol) in dry toluene (60 mL) at room temperature under nitrogen atmosphere. The reaction mixture was then heated at 130° C. for 2.5 hr, removed from the oil bath, and allowed to cool to room temperature. The condenser walls were rinsed with 10 mL of toluene and the washing was added to the reaction mixture. The toluene and excess thionyl chloride were removed under reduced pressure and the light yellow liquid was held under vacuum pump for 45 min. This pale yellow-colored oil weighed (9.98 g) and represented a yield of 62% ...
example 3
Preparation of (S)-2-(2-Methoxynaphthalene-6-yl)propanoyl Chloride [naproxen acid chloride]
[0042]
[0043]By the method and equipment described in Example 2, 25.8 g (0.22 mol) of thionyl chloride was added via glass syringe to 10.0 g (0.043 mol) of (S)-2-(2-methoxynaphthalene-6-yl)propanoic acid (also known as naproxen) in 60 mL of dry toluene at room temperature under nitrogen atmosphere. The reaction mixture was then heated at 130° C. for 2.5 hr and worked up as described. Evaporation in vacuo began to precipitate a light yellow solid to which 40 mL of anhydrous hexane were added. The hexane and the suspended yellow solid were stirred vigorously under dry nitrogen atmosphere for 10 min and filtered to obtain, after vacuum drying, 9.85 g of light yellow acid chloride. Dry hexane was added (40 mL), and stirred for 10 min. under nitrogen. This crude product was stored in a nitrogen flushed glass vial and used for the coupling reaction without additional purification. The yield of 79%, f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| lipophilic | aaaaa | aaaaa |
| hydrolyzable | aaaaa | aaaaa |
| HPLC chromatogram | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com